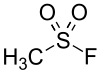
Methanesulfonyl fluoride
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Methanesulfonyl fluoride[1] | |||
| Other names
Fumette, Mesyl fluoride | |||
| Identifiers | |||
| 558-25-8 | |||
| 3D model (Jmol) | Interactive image | ||
| Abbreviations | MSF | ||
| ChemSpider | 10734 | ||
| ECHA InfoCard | 100.008.358 | ||
| EC Number | 209-192-2 | ||
| MeSH | methanesulfonyl+fluoride | ||
| PubChem | 11207 | ||
| RTECS number | PB2975000[2] | ||
| UN number | UN3389 | ||
| |||
| |||
| Properties | |||
| CH3FO2S | |||
| Molar mass | 98.09 g·mol−1 | ||
| Appearance | liquid | ||
| Odor | pungent | ||
| Density | 1.427 g/mL[3] | ||
| Boiling point | 123 to 124 °C (253 to 255 °F; 396 to 397 K)[3] | ||
| Reacts | |||
| Refractive index (nD) |
1.360 [2] | ||
| Hazards | |||
| Main hazards | Corrosive Highly toxic | ||
| GHS pictograms |   | ||
| H300, H330, H314, H318 | |||
| P301+310, P303+361+353, P304+340, P305+351+338, P320, P330, P405, P501 | |||
| R-phrases | R26/28 R34 | ||
| S-phrases | S9 S26 S28 S36/37/39 S45 S60 | ||
| Related compounds | |||
| Other anions |
Methanesulfonyl chloride | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Methanesulfonyl fluoride (MSF) has long been known to be a potent inhibitor of acetylcholinesterase AChE, the enzyme that regulates acetylcholine, an important neurotransmitter in both the central and peripheral nervous systems.[4][5]
Technical and physical properties
MSF is a clear, colorless to yellowish hygroscopic liquid (attracts and holds water by absorption or adsorption). It is corrosive and highly toxic. It is an oxydiaphoric inhibitor (acid-transferring inhibitor)[6] of the enzyme acetylcholinesterase.[6][3] MSF, which is a liquid at room temperature, has a vapor pressure of 19.2 mmHg, slightly more volatile than water which has a vapor pressure of 18.8 mmHg at 21 °C. This vapor has an LCt50 (lethal concentration, 50% death rate in a given time) in rats of between 4 and 5 parts per million (ppm) during one hour of exposure or between 1 and 1.2 ppm during 7 hours of exposure. MSF produced no subtle biological effects from direct action of MSF independent of its ability to inhibit cholinesterase. Repeated exposures to 1/10 of the LCt50 did not produce overt systemic toxicity or significant pathology.[7] MSF can also cause severe skin burns and serious eye damage, if contact is made. It is a lachrymator and its vapor causes tears in eyes.[8][2]
Methanesulfonyl fluoride has a pungent odor. It undergoes decomposition on heating to liberate additional toxic fumes of fluorides and sulfur oxides (SOx).[9]
Synthesis
A typical synthesis is to treat methanesulfonyl chloride with potassium fluoride or potassium bifluoride in water and then steam distill the product out.[10]
Therapeutic study
Animal studies have shown that MSF-induced inhibition of AChE is highly selective for the brain when it is studied in vivo. MSF is an irreversible inhibitor of AChE and its inhibition of AChE is only overcome by de novo synthesis of new AChE in each tissue. The recovery of AChE activity in the brain is less than one-tenth as rapid as AChE recovery in the gastrointestinal system, allowing very high accumulated AChE inhibition in the brain with clinically insignificant AChE inhibition in peripheral tissues.[11][12]The high selectivity of MSF for inhibition in the brain has suggested that it could be used as a treatment for dementia of the Alzheimer type,[11] that it is effective in reducing the persistent cognitive deficit after stroke,[13] and it is highly effective in reducing normal age-related memory impairment, making aged rats perform as well as younger rats. [14]
Methanesulfonyl fluoride has been tested successfully in three human clinical trials as a treatment for Alzheimer's dementia. Two Phase I clinical trials testing for safety of MSF in humans at the doses proposed for treating dementia have shown that it is well tolerated and relatively free of the side effects of nausea, vomiting, and diarrhea that are produced by the current cholinesterase inhibitors used for the treatment of Alzheimer's dementia.[15] Furthermore, a Phase II, clinical trial testing both the safety and the efficacy of MSF found that it was well tolerated in the aged patients, and it was highly effective in reducing the symptoms of Alzheimer's dementia.[15] US Patent has been issued for the use of MSF for the treatment of dementia.[16]
References
- ↑ "methanesulfonyl fluoride - Compound Summary (CID 11207)". PubChem. Retrieved July 25, 2012.
- 1 2 3 "H53460 Methanesulfonyl fluoride, 98%". Retrieved July 25, 2012.
- 1 2 3 "METHANESULFONYL FLUORIDE". chembook. Retrieved July 25, 2012.
- ↑ Myers, D. K.; Kemp, A. (1954). "Inhibition of Esterases by the Fluorides of Organic Acids". Nature. 173 (4392): 33–4. doi:10.1038/173033a0. PMID 13119739.
- ↑ Kitz, R; Wilson, IB (1962). "Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase". The Journal of Biological Chemistry. 237: 3245–9. PMID 14033211.
- 1 2 Kitz, R; Wilson, IB (1963). "Acceleration of the rate of reaction of methanesulfonyl fluoride and acetylcholinesterase by substituted ammonium ions". The Journal of Biological Chemistry. 238: 745–8 http://www.jbc.org/content/238/2/745.full.pdf#page=1&view=FitH free, automatic full text pdf download, to read offline. PMID 14033210.
- ↑ Hine, C.H.; Lyons, J.; Eisenlord, G.H.; Wright, J.A.; Long, J.E. (1979). "Three-month inhalation exposure study with methane sulfonylfluoride". American Industrial Hygiene Association Journal. 40 (11): 986–92. doi:10.1080/15298667991430596. PMID 532785.
- ↑ MSDS, Fisher Scientific
- ↑ Extremely Hazardous Substances: Superfund Chemical Profiles. William Andrew. 1988. p. 997. ISBN 978-0-8155-1166-3. Retrieved 25 July 2012.
- ↑ Gramstad, T.; Haszeldine, R. N. (1956). "33. Perfluoroalkyl derivatives of sulphur. Part IV. Perfluoroalkanesulphonic acids". Journal of the Chemical Society (Resumed): 173–180. doi:10.1039/JR9560000173.
- 1 2 Moss, D. E.; Rodriguez, L. A.; Herndon, W. C.; Vincenti, S. P.; Camarena, M. L. (1986). "Alzheimer's and Parkinson's Disease". Advances in Behavioral Biology. Advances in Behavioral Biology. 29: 551–556. doi:10.1007/978-1-4613-2179-8_62. ISBN 978-1-4612-9283-8.
|chapter=ignored (help) - ↑ Moss, D.E.; Fariello, Ruggero G.; Sahlmann, Jörg; Sumaya, Isabel; Pericle, Federica; Braglia, Enrico (2012). "A Randomized Phase I Study of Methanesulfonyl Fluoride, an Irreversible Cholinesterase Inhibitor, for the Treatment of Alzheimer's Disease". British Journal of Clinical Pharmacology. 75 (5): n/a. doi:10.1111/bcp.12018.
- ↑ Borlongan, Cesario V.; Sumaya, Isabel C.; Moss, Donald E. (2005). "Methanesulfonyl fluoride, an acetylcholinesterase inhibitor, attenuates simple learning and memory deficits in ischemic rats". Brain Research. 1038 (1): 50–8. doi:10.1016/j.brainres.2005.01.028. PMID 15748872.
- ↑ Malin, David H.; Plotner, Robert E.; Radulescu, Sarah J.; Ferebee, Robert N.; Lake, J.Ronald; Negrete, Pilar G.; Schaefer, Peggy J.; Crothers, Marie K.; Moss, Donald E. (1993). "Chronic methanesulfonyl fluoride enhances one-trial per day reward learning in aged rats". Neurobiology of Aging. 14 (4): 393–5. doi:10.1016/0197-4580(93)90127-W. PMID 8367021.
- 1 2 Moss, DE; Berlanga, P; Hagan, MM; Sandoval, H; Ishida, C (1999). "Methanesulfonyl fluoride (MSF): A double-blind, placebo-controlled study of safety and efficacy in the treatment of senile dementia of the Alzheimer type". Alzheimer Disease and Associated Disorders. 13 (1): 20–5. doi:10.1097/00002093-199903000-00003. PMID 10192638.
- ↑ US 5798392, Moss; Donald Eugene, "Sulfonyl fluorides for the treatment of Alzheimer's disease", issued 1998-08-25