Non-Newtonian fluid
A non-Newtonian fluid is a fluid with properties that are different in any way from those of Newtonian fluids. Most commonly, the viscosity (the measure of a fluid's ability to resist gradual deformation by shear or tensile stresses) of non-Newtonian fluids is dependent on shear rate or shear rate history. Some non-Newtonian fluids with shear-independent viscosity, however, still exhibit normal stress-differences or other non-Newtonian behavior. Many salt solutions and molten polymers are non-Newtonian fluids, as are many commonly found substances such as ketchup, custard, toothpaste, starch suspensions, maizena, paint, blood, and shampoo.
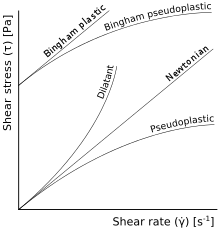
In a Newtonian fluid, the relation between the shear stress and the shear rate is linear, passing through the origin, the constant of proportionality being the coefficient of viscosity. In a non-Newtonian fluid, the relation between the shear stress and the shear rate is different and can even be time-dependent (Time Dependent Viscosity). Therefore, a constant coefficient of viscosity cannot be defined.
Although the concept of viscosity is commonly used in fluid mechanics to characterize the shear properties of a fluid, it can be inadequate to describe non-Newtonian fluids. They are best studied through several other rheological properties that relate stress and strain rate tensors under many different flow conditions—such as oscillatory shear or extensional flow—which are measured using different devices or rheometers. The properties are better studied using tensor-valued constitutive equations, which are common in the field of continuum mechanics.
Types of non-Newtonian behaviour
Summary
| Viscoelastic | Kelvin material, Maxwell material | "Parallel" linearstic combination of elastic and viscous effects[1] | Some lubricants, whipped cream, Silly Putty |
| Time-dependent viscosity | Rheopecty | Apparent viscosity increases with duration of stress | Synovial fluid, printer ink, gypsum paste |
| Thixotropic | Apparent viscosity decreases with duration of stress[1] | Yogurt, xanthan gum solutions, aqueous iron oxide gels, gelatin gels, pectin gels, hydrogenated castor oil, some clays (including bentonite, and montmorillonite), carbon black suspension in molten tire rubber, some drilling muds, many paints, many floc suspensions, many colloidal suspensions | |
| Time-independent viscosity | Shear thickening (dilatant) | Apparent viscosity increases with increased stress[2] | Suspensions of corn starch in water |
| Shear thinning (pseudoplastic) | Apparent viscosity decreases with increased stress[3][4] | Nail polish, whipped cream, ketchup, molasses, syrups, paper pulp in water, latex paint, ice, blood, some silicone oils, some silicone coatings, sand in water | |
| Generalized Newtonian fluids | Viscosity is constant. Stress depends on normal and shear strain rates and also the pressure applied on it |
Blood plasma, custard, water |
Shear thickening fluid
The viscosity of a shear thickening fluid, or dilatant fluid, appears to increase when the shear rate increases. Corn starch dissolved in water ("oobleck", see below) is a common example: when stirred slowly it looks milky, when stirred vigorously it feels like a very viscous liquid.
Shear thinning fluid
A familiar example of the opposite, a shear thinning fluid, or pseudoplastic fluid, is wall paint: The paint should flow readily off the brush when it is being applied to a surface but not drip excessively. Note that all thixotropic fluids are extremely shear thinning, but they are significantly time dependent, whereas the colloidal "shear thinning" fluids respond instantaneously to changes in shear rate. Thus, to avoid confusion, the latter classification is more clearly termed pseudoplastic.
Another example of a shear thinning fluid is blood. This application is highly favoured within the body, as it allows the viscosity of blood to decrease with increased shear strain rate.
Bingham plastic
Fluids that have a linear shear stress/shear strain relationship require a finite yield stress before they begin to flow (the plot of shear stress against shear strain does not pass through the origin). These fluids are called Bingham plastics. Several examples are clay suspensions, drilling mud, toothpaste, mayonnaise, chocolate, and mustard. The surface of a Bingham plastic can hold peaks when it is still. By contrast Newtonian fluids have flat featureless surfaces when still.
Rheopectic or anti-thixotropic
There are also fluids whose strain rate is a function of time. Fluids that require a gradually increasing shear stress to maintain a constant strain rate are referred to as rheopectic. An opposite case of this is a fluid that thins out with time and requires a decreasing stress to maintain a constant strain rate (thixotropic).
Examples
Many common substances exhibit non-Newtonian flows. These include:[5]
- Soap solutions, cosmetics and toothpaste
- Food such as butter, cheese, jam, ketchup, mayonnaise, soup, taffy, and yogurt
- Natural substances such as magma, lava, gums, and extracts such as vanilla extract
- Biological fluids such as blood, saliva, semen, mucus and synovial fluid
- Slurries such as cement slurry and paper pulp, emulsions such as mayonnaise, and some kinds of dispersions
Oobleck

An inexpensive, non-toxic example of a non-Newtonian fluid is a suspension of starch (e.g. cornstarch) in water, sometimes called "Oobleck," "ooze," or "magic mud" (1 part of water to 1.5–2 parts of corn starch).[7][8][9] Uncooked cornflour has the same properties. The name "oobleck" is derived from the Dr. Seuss book Bartholomew and the Oobleck.[7]
Because of its properties, oobleck is often used in demonstrations that exhibit its unusual behavior. A person may walk on a large tub of oobleck without sinking due to its shear thickening properties, given the individual moves quickly enough to provide enough force with each step to cause the thickening. Also, if oobleck is placed on a large subwoofer driven at a sufficiently high volume, it will thicken and form standing waves in response to low frequency sound waves from the speaker.
Flubber
Flubber is a non-Newtonian fluid, easily made from polyvinyl alcohol–based glues and borax. It flows under low stresses but breaks under higher stresses and pressures. This combination of fluid-like and solid-like properties makes it a Maxwell fluid. Its behaviour can also be described as being viscoplastic or gelatinous.[10]
Chilled caramel topping
Another example of this is chilled caramel ice cream topping (so long as it incorporates hydrocolloids such as carrageenan and gellan gum). The sudden application of force—by stabbing the surface with a finger, for example, or rapidly inverting the container holding it—causes the fluid to behave like a solid rather than a liquid. This is the "shear thickening" property of this non-Newtonian fluid. More gentle treatment, such as slowly inserting a spoon, will leave it in its liquid state. Trying to jerk the spoon back out again, however, will trigger the return of the temporary solid state.[11]
Silly Putty
Silly Putty is a silicone polymer based suspension which will flow, bounce, or break depending on strain rate.
Plant resin
Plant resin is a viscoelastic solid polymer. When left in a container, it will flow slowly as a liquid to conform to the contours of its container. If struck with greater force, however, it will shatter as a solid.
Ketchup
Ketchup is a shear thinning fluid.[2][12] Shear thinning means that the fluid viscosity decreases with increasing shear stress. In other words, fluid motion is initially difficult at slow rates of deformation, but will flow more freely at high rates.
See also
- Bingham plastic
- Caramel
- Complex fluid
- Dilatant
- Dissipative particle dynamics
- Generalized Newtonian fluid
- Herschel–Bulkley fluid
- Navier–Stokes equations
- Newtonian fluid
- Pseudoplastic
- Quicksand
- Rheology
- Superfluids
- Weissenberg effect
References
- 1 2 Tropea, Cameron; Yarin, Alexander L.; Foss, John F. (2007). Springer handbook of experimental fluid mechanics. Springer. pp. 661, 676. ISBN 978-3-540-25141-5.
- 1 2 Garay, Paul N. (1996). Pump Application Desk Book (3rd ed.). Prentice Hall. p. 358. ISBN 978-0-88173-231-3.
- ↑ Rao, M. A. (2007). Rheology of Fluid and Semisolid Foods: Principles and Applications (2nd ed.). Springer. p. 8. ISBN 978-0-387-70929-1.
- ↑ Schramm, Laurier L. (2005). Emulsions, Foams, and Suspensions: Fundamentals and Applications. Wiley VCH. p. 173. ISBN 978-3-527-30743-2.
- ↑ Chhabra, R.P. (2006). Bubbles, Drops, and Particles In Non-Newtonian Fluids. (2nd ed.). Hoboken: Taylor & Francis Ltd. pp. 9–10. ISBN 1420015389.
- ↑ This demonstration of oobleck is a popular subject for YouTube videos, such as this.
- 1 2 Oobleck: The Dr. Seuss Science Experiment
- ↑ "Outrageous Ooze". Exploratorium.
- ↑ Rupp, Rebecca. "Magic Mud and Other Great Experiments". The Complete Home Learning Source Book. pp. 235–236.
- ↑ Glurch Meets Oobleck. Iowa State University Extension.
- ↑ Barra, Giuseppina (2004). The Rheology of Caramel (Ph.D.). University of Nottingham.
- ↑ Cartwright, Jon (2 September 2011). "Microscopy reveals why ketchup squirts". Chemistry World. Royal Society of Chemistry.
External links
| Wikimedia Commons has media related to Non-Newtonian Fluid. |
- Classical experiments with Non-Newtonian fluids by the National Committee for Fluid Mechanics on YouTube