PYR-41
 | |
| Names | |
|---|---|
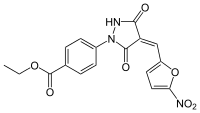
| IUPAC name
4[4-(5-nitro-furan-2-ylmethylene)-3,5-dioxo-pyrazolidin-1-yl]-benzoic acid ethyl ester | |
| Identifiers | |
| 418805-02-4 | |
| 3D model (Jmol) | Interactive image |
| ECHA InfoCard | 100.213.089 |
| PubChem | 5335621 |
| |
| Properties | |
| C17H13N3O7 | |
| Molar mass | 371.3 g/mol |
| Density | 1.5±0.1 g/cm3 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
PYR-41 is the first cell permeable inhibitor of ubiquitin-activating enzyme E1,irreversibly inhibits ubiquitin-activating enzyme activity.It is a pyrazone compound that showed little or no activity on E2,E3.[1]
Unexpectedly, despite of ubiquitylation inhibition, PYR-41 also enhances total sumoylation in cells.[2]
PYR41 also blocks the downstream ubiquitination and ubiquitination-dependent protein degradation or other ubiquitination-mediated cellular activities. Besides, PYR-41 inhibits degradation of p53, a tumour suppressor and also activates the transcription activity of it.[3] PYR-41 and related pyrazones selectively kill transformed p53 expressing cells, suggesting that E1 inhibitors may be potential therapeutics in cancer.
References
- ↑ "biological activity of PYR41 in selleckchem". selleck chemicals LLC. Sep 25, 2014.
- ↑ Kapuria V, Peterson LF, Showalter HD, et al. (Aug 15, 2011). "Protein cross-linking as a novel mechanism of action of a ubiquitin-activating enzyme inhibitor with anti-tumor activity.". Biochem Pharmacol. 82 (4): 341–349. doi:10.1016/j.bcp.2011.05.012. PMID 21621524.
- ↑ Chen C, et al. (Jun 2014). "Ubiquitin-activating enzyme E1 inhibitor PYR-41 attenuates angiotensin II-induced activation of dendritic cells via the IκBa/NF-κB and MKP1/ERK/STAT1 pathways.". Immunology. 142 (2): 307–319. doi:10.1111/imm.12255. PMID 24456201.
The main article for this category is Benzoate.
| Wikimedia Commons has media related to Benzoates. |
This article is issued from Wikipedia - version of the 9/1/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.