Palladium-catalyzed coupling reactions
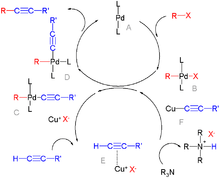
Palladium-catalyzed coupling reactions comprise a family of cross-coupling reactions that employ palladium complexes as catalysts. It is an active area of research and applications in homogeneous catalyst. In 2010, the Nobel Prize in Chemistry was awarded to Richard F. Heck, Ei-ichi Negishi and Akira Suzuki for their work on palladium-catalyzed cross couplings in organic synthesis.[1][2]
Examples
The reactions generally obey the following stoichiometry:
- X-R + M-R' → MX + R-R'
Variations are based on the identity of X-R (often an aryl bromide) and M-R'. Often the reactions generate salts, or salt-like products (zinc halides, tin halides, silicon halides)
- Negishi coupling between an organohalide and an organozinc compound
- Heck reaction between alkenes and aryl halides
- Suzuki reaction between aryl halides and boronic acids
- Stille reaction between organohalides and organotin compounds
- Hiyama coupling between organohalides and organosilicon compounds
- Sonogashira coupling between aryl halides and alkynes, with copper(I) iodide as a co-catalyst
- The Buchwald-Hartwig amination of an aryl halide with an amine, extended to aryl halide with phenol and thiol
- The Kumada coupling of grignards and aryl or vinyl halides
- The Heck-Matsuda Reaction of an arenediazonium salt with an alkene
Catalysts
Typical palladium catalysts used include the following compounds:
- palladium acetate, Pd(OAc)2
- tetrakis(triphenylphosphine)palladium(0), Pd(PPh3)4
- bis(triphenylphosphine)palladium(II) dichloride, PdCl2(PPh3)2
- [1,1'-bis(diphenylphosphino)ferrocene]palladium(II) dichloride
Some of these catalysts are really pro-catalysts, that become activated in situ. For example, PdCl2(PPh3)2 is reduced to a Pd(0) complex or transmetalated to a Pd(II) aryl complex before it participates in the catalytic cycle.
Operating conditions
Unoptimized reactions typically use 10-15 mol% of palladium. In optimized reactions, catalyst loadings can be on the order of 0.1 mol % or below. Palladium nano clusters have been found to catalyze coupling reactions with catalyst loadings as low as parts per billion, however such systems typically do not maintain catalytic activity as long as well defined ligated catalysts.[3] Many exotic ligands and chiral catalysts have been reported, but they are largely not available commercially, and do not find widespread use. Much work is being done on replacing the phosphine ligands with other classes, such as Arduengo-type carbene complexes, as the phosphine ligands are typically oxygen sensitive (easily oxidized) and must be handled under an inert atmosphere.[4] Phosphines are labile, sometimes requiring additional ligand. For example, Pd(PPh3)4 would be supplemented with PPh3 to keep the palladium coordinated despite loss of the labile phosphine ligands.
A concern with the use of palladium in the preparation of pharmaceuticals is that traces of the toxic heavy metal will remain in the product. Column chromatography can be used, but solid-phase metal scavengers (ion exchange resins and derivatives of silica gel) promise more efficient separation.
See also
References
- ↑ http://nobelprize.org/nobel_prizes/chemistry/laureates/2010/
- ↑ PALLADIUM-CATALYZED CROSS COUPLINGS IN ORGANIC SYNTHESIS details of reactions
- ↑ Dong, Zhongmin; Ye, Zhibin (2014-11-03). "Reusable, Highly Active Heterogeneous Palladium Catalyst by Convenient Self-Encapsulation Cross-Linking Polymerization for Multiple Carbon Cross-Coupling Reactions at ppm to ppb Palladium Loadings". Advanced Synthesis & Catalysis. 356 (16): 3401–3414. doi:10.1002/adsc.201400520. ISSN 1615-4169.
- ↑ Fortman, George C.; Nolan, Steven P. "N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: a perfect union". Chemical Society Reviews. 40 (10). doi:10.1039/c1cs15088j.