Pentagonal bipyramidal molecular geometry
| Pentagonal bipyramidal molecular geometry | |
|---|---|
 | |
| Examples | IF7 |
| Point group | D5h |
| Steric number | 7 |
| Coordination number | 7 |
| Bond angle(s) | 72°, 90° |
| μ (Polarity) | 0 |
In chemistry, a pentagonal bipyramid (or dipyramid) is a molecular geometry with one atom at the centre with seven ligands at the corners of a pentagonal dipyramid. A perfect pentagonal bipyramid belongs to the molecular point group D5h.
The pentagonal bipyramid is a case where bond angles surrounding an atom are not identical (see also trigonal bipyramidal molecular geometry).[1] Other seven coordinate geometries include the mono-capped octahedron and mono-capped trigonal prism. A variety of transition metal complexes adopt heptacoordination, but the symmetry is usually lower than D5h.
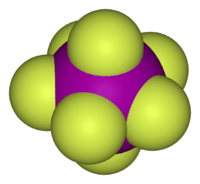
Structure of iodine heptafluoride, an example of a molecule with the pentagonal-bipyramidal coordination geometry.
Examples
- Iodine heptafluoride (IF7) with 7 bonding groups
- Peroxo chromium(IV) complexes, e.g. [Cr(O2)2(NH3)3] where the peroxo groups occupy four of the planar positions.
References
- ↑ Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
External links
- – Images of IF7
- 3D Chem – Chemistry, Structures, and 3D Molecules
- IUMSC – Indiana University Molecular Structure Center
This article is issued from Wikipedia - version of the 5/31/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.