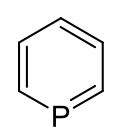
Phosphorine
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
Phosphinine[1] | |||
| Other names
Phosphabenzene | |||
| Identifiers | |||
| 289-68-9 | |||
| 3D model (Jmol) | Interactive image Interactive image | ||
| ChemSpider | 109668 | ||
| MeSH | Phosphinine | ||
| PubChem | 123046 | ||
| |||
| |||
| Properties | |||
| C5H5P | |||
| Molar mass | 96.07 g·mol−1 | ||
| Related compounds | |||
| Related -ines |
Arsabenzene | ||
| Related compounds |
Phosphole | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Phosphorine (IUPAC name: phosphinine) is a heavier element analog of pyridine, containing a phosphorus atom instead of an aza- moiety. It is also called phosphabenzene and belongs to the phosphaalkene class. It is a colorless liquid that is mainly of interest in research.
Phosphorine is generally stable against air and moisture and can be handled without special air-free techniques. In contrast, silabenzene, a related heavy-element analogue of benzene, is not only air- and moisture-sensitive but also thermally unstable without extensive steric protection.
History
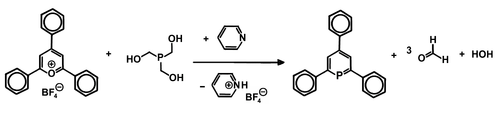
The first phosphorine to be isolated is 2,4,6-triphenylphoshorine. It was synthesized by Gottfried Märkl in 1966 by condensation of the corresponding pyrylium salt and phosphine or its equivalent ( P(CH2OH)3 and P(SiMe3)3).[2]
The parent (unsubstituted) phosphorine was reported by Arthur J. Ashe III in 1971.[3][4] Ring-opening approaches have been developed from phospholes.[5]
Structure, bonding, and properties
Structural studies by electron diffraction reveal that phosphorine is a planar aromatic compound with 88% of the aromaticity of that of benzene. Potentially relevant to its high aromaticity are the well matched electronegativities of phosphorus (2.1) and carbon (2.5). The P-C bond length is 173 pm and the C-C bond lengths center around 140 pm and show little variation.[6]
Although phosphorine and pyridine are structurally similar, phosphorines are far less basic. The pKa's of C5H5PH+ and C5H5NH+ are respectively -16.1 and 5.2.[5] Methyl lithium adds to phosphorus in phosphorine whereas it adds to the 2-position of pyridine.[7]
Phosphorine undergoes electrophilic substitution reactions like ordinary aromatic compounds: bromination, acylation, and so on.
Coordination chemistry
Coordination complexes bearing phosphorine as a ligand are known. Phosphorines can bind to metals through phosphorus center. Complexes of the diphospha analogue of bipyridine are known. Phosphorines also form pi-complexes, illustrated by V(η6-C5H5P)2.[5]
See also
- 6-membered aromatic rings with one carbon replaced by another group: borabenzene, benzene, silabenzene, germabenzene, stannabenzene, pyridine, phosphorine, arsabenzene, pyrylium salt
References
- ↑ "Phosphate-Binding Proteolipid - Compound Summary". The PubChem Project. USA: National Center of Biotechnology Information.
- ↑ G. Märkl, 2,4,6-Triphenylphosphabenzol in Angewandte Chemie 78, 907–908 (1966)
- ↑ Ashe, A. J. (1971). "Phosphabenzene and Arsabenzene". Journal of the American Chemical Society. 93 (13): 3293–3295. doi:10.1021/ja00742a038.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 544. ISBN 0-08-037941-9.
- 1 2 3 François Mathey "Phosphorus Heterocycles" in Modern Heterocyclic Chemistry, First Edition, edited by Julio Alvarez-Builla, Juan Jose Vaquero, José Barluenga, Wiley-VCH, Weinheim, 2011. doi:10.1002/9783527637737.ch23.
- ↑ László Nyulászi "Aromaticity of Phosphorus Heterocycles" Chem. Rev., 2001, volume 101, pp 1229–1246. doi:10.1021/cr990321x
- ↑ Ashe III, Arthur J.; Smith, Timothy W. "The reaction of phosphabenzene, arsabenzene and stibabenzene with methyllithium." Tetrahedron Letters 1977, volume 18, pp. 407-410. doi:10.1016/S0040-4039(01)92651-6
- Quin, L. D. (2000). A Guide to Organophosphorus Chemistry. Wiley-Interscience. ISBN 0-471-31824-8.