Pioneer factor
Pioneer factors are transcription factors that can directly bind condensed chromatin. They can have positive and negative effects on transcription and are important in recruiting other transcription factors and histone modification enzymes as well as controlling DNA methylation. They were first discovered in 2002 as factors capable of binding to target sites on nucleosomal DNA in compacted chromatin and endowing competency for gene activity during hepatogenesis.[1] Pioneer factors are involved in initiating cell differentiation and activation of cell-specific genes. This property is observed in fork head box (FOX), Groucho TEL, and in Gal4 transcription factors.[2]
The eukaryotic cell condenses its genome into tightly packed chromatin and nucleosomes. This ability saves space in the nucleus for only actively transcribed genes and hides unnecessary or detrimental genes from being transcribed. Access to these condensed regions is done by chromatin remodelling by either balancing histone modifications or directly with pioneer factors that can loosen the chromatin themselves or as a flag recruiting other factors. Pioneer factors are not necessarily required for assembly of the transcription apparatus and may dissociate after being replaced by other factors.
Active rearrangement
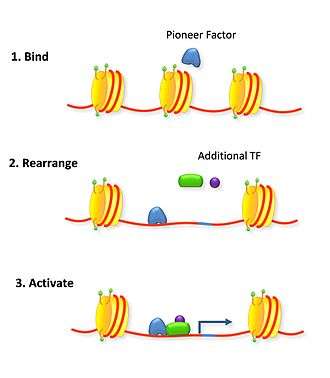
Pioneer factors can also actively affect transcription by directly opening up condensed chromatin in an ATP-independent process. This is a common trait of fork head factors as they contain a winged helix DNA-binding domain that mimics the DNA-binding domain of the linker H1 histone.[3] The similarity to histone H1 explains how it is able to bind chromatin by interacting with the major groove of only the one available side of DNA wrapped around a nucleosome.[3][4] Fork head domains also have a helix that confers sequence specificity unlike linker histone.[3][5] The C terminus is associated with higher mobility around the nucleosome than linker histone, displacing it and rearranging nucleosomal landscapes effectively.[4] This active re-arrangement of the nucleosomes allows for other transcription factors to bind the available DNA. In thyroid cell differentiation FoxE binds to compacted chromatin of the thyroid peroxidase promoter and opens it for NF1 binding.[6]
Passive factors
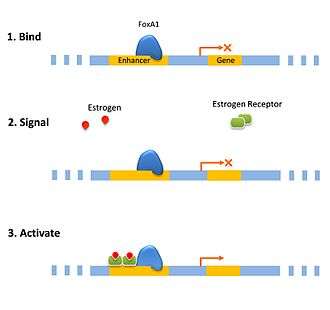
Pioneer factors can function in a passively acting as a bookmark for the cell to recruit other transcription factors to specific genes in condensed chromatin. This can be important for priming the cell for a rapid response as the enhancer is already bound by a pioneer transcription factor giving it a head start towards assembling the transcription preinitiation complex. Hormone responses are often quickly induced in the cell using this priming method such as with the estrogen receptor.[7] Another form of priming is when an enhancer is simultaneously bound by activating and repressing pioneer factors. This balance can be tipped by dissociation of one of the factors. In hepatic cell differentiation the activating pioneer factor FOXA1 recruites a repressor, grg3, that prevents transcription until the repressor is down-regulated later on in the differentiation process.[8]
In a direct role pioneer factors can bind an enhancer and recruit activation complex that will modify the chromatin directly. The change in the chromatin changes the affinity, decreasing the affinity of the pioneer factor such that it is replaced by a transcription factor that has a higher affinity. This is a mechanism for the cell to switch a gene on was observed with glucocorticoid receptor recruiting modification factors that then modify the site to bind activated estrogen receptor which was coined as a “bait and switch” mechanism.[9]
Epigenetic effects
Pioneer factors can exhibit their greatest range of effects on transcription through the modulation of epigenetic factors by recruiting activating or repressing histone modification enzymes and controlling CpG methylation by protecting specific cysteine residues. This has effects on controlling the timing of transcription during cell differentiation processes.
Histone modification
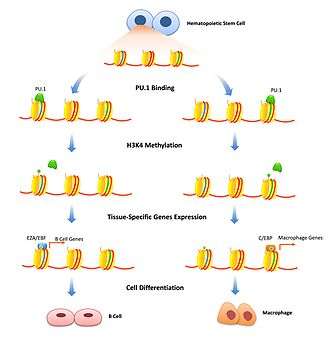
Histone modification is a well-studied mechanism to transiently adjust chromatin density. Pioneer factors can play a role in this by binding specific enhancers and flagging histone modification enzymes to that specific gene. Repressive pioneer factors can inhibit transcription by recruiting factors that modify histones that further tighten the chromatin. This is important to limit gene expression to specific cell types and has to be removed only when cell differentiation begins. FoxD3 has been associated as a repressor of both B-cell and melanocytic cell differentiation pathways, maintaining repressive histone modifications where bound, that have to be overcome to start differentiation.[10][11] Pioneer factors can also be associated with recruiting transcription-activating histone modifications. Enzymes that modify H3K4 with mono and di-methylation are associated with increasing transcription and have been shown to bind pioneer factors.[7] In B cell differentiation PU.1 is necessary to signal specific histones for activating H3K4me1 modifications that differentiate hematopoietic stem cells into either the B-cell or macrophage lineage.[12] FoxA1 binding induces HSK4me2 during neuronal differentiation of pluripotent stem cells [13] as well as the loss of DNA methylation.[14]
DNA methylation
Pioneer factors can also affect transcription and differentiation through the control of DNA methylation. Pioneer factors that bind to CpG islands and cysteine residues block access to methyltransferases. Many eukaryotic cells have CpG islands in their promoters that can be modified by methylation having adverse effects on their ability to control transcription.[15] This phenomenon is also present in promoters without CpG islands where single cysteine residues are protected from methylation until further cell differentiation. An example is FoxD3 preventing methylation of a cysteine residue in Alb1 enhancer, acting as a place holder for FoxA1 later in hepatic [16] as well as in CpG islands of genes in chronic lymphocytic leukemia.[17] For stable control of methylation state the cysteine residues are covered during mitosis, unlike most other transcription factors, to prevent methylation. Studies have shown that during mitosis 15% of all interphase FoxA1 binding sites were bound.[18] The protection of cysteine methylation can be quickly removed allowing for rapid induction when a signal is present.
Other pioneer factors
A well studied pioneer factor family is the Goucho-related (Gro/TLE/Grg) transcription factors that often have a negative effect on transcription. These chromatin binding domains can span up to 3-4 nucleosomes. These large domains are scaffolds for further protein interactions and also modify the chromatin for other pioneer factors such as FoxA1 which has been shown to bind to Grg3.[19] Transcription factors with zinc finger DNA binding domains, such as the GATA family and glucocorticoid receptor.[7] The zinc finger domains do not appear to bind nucleosomes well and can be displaced by FOX factors.[18]
Role in cancer
The ability of pioneer factors to respond to extracellular signals to differentiate cell type has been studied as a potential component of hormone-dependent cancers. Hormones such as estrogen and IGFI are shown to increase pioneer factor concentration leading to a change in transcription.[20] Known pioneer factors such as FoxA1, PBX1, TLE, AP2ɣ, GATA factors 2/3/4, and PU.1 have been associated with hormone-dependent cancer . FoxA1 is necessary for estrogen and androgen mediated hepatocarcinogenesis and is a defining gene for ER+ luminal breast cancer, as is another pioneer factor GATA3.[7][20] FOXA1 particularly is expressed in 90% of breast cancer metastases and 89% of metastic prostate cancers.[20][21] In the breast cancer cell line, MCF-7, it was found that FoxA1 was bound to 50% of estrogen receptor binding sites independent of estrogen presence. High expression of pioneer factors is associated with poor prognosis with the exception of breast cancer where FoxA1 is associated with a stronger outcome.[20]
The correlation between pioneer factors and cancer has led to prospective therapeutic targeting. In knockdown studies in the MCF-7 breast cancer cell line it was found that decreasing pioneer factors FoxA1 and AP2ɣ decreased ER signalling.[2][20] Other fork head proteins have been associated with cancer, including FoxO3 and FoxM that repress the cell survival pathways Ras and PPI3K/AKT/IKK.[22] Drugs such as Paclitaxel, Imatinib, and doxorubicin which activate FoxO3a or its targets are being used. Modification to modulate related factors with pioneer activity is a topic of interest in the early stages as knocking down pioneer factors may have toxic effects through alteration of the lineage pathways of healthy cells.[20]
References
- ↑ Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS (November 2002). "Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4". Molecular Cell. 9 (2): 279–89. doi:10.1016/S1097-2765(02)00459-8. PMID 11864602.
- 1 2 Magnani L, Eeckhoute J, Lupien M (November 2011). "Pioneer factors: directing transcriptional regulators within the chromatin environment". Trends Genet. 27 (11): 465–74. doi:10.1016/j.tig.2011.07.002. PMID 21885149.
- 1 2 3 Clark KL, Halay ED, Lai E, Burley SK (July 1993). "Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5". Nature. 364 (6436): 412–20. doi:10.1038/364412a0. PMID 8332212.
- 1 2 Zaret KS, Caravaca JM, Tulin A, Sekiya T (2010). "Nuclear mobility and mitotic chromosome binding: similarities between pioneer transcription factor FoxA and linker histone H1". Cold Spring Harb. Symp. Quant. Biol. 75: 219–26. doi:10.1101/sqb.2010.75.061. PMID 21502411.
- ↑ Sekiya T, Muthurajan UM, Luger K, Tulin AV, Zaret KS (April 2009). "Nucleosome-binding affinity as a primary determinant of the nuclear mobility of the pioneer transcription factor FoxA". Genes Dev. 23 (7): 804–9. doi:10.1101/gad.1775509. PMC 2666343
 . PMID 19339686.
. PMID 19339686. - ↑ Cuesta I, Zaret KS, Santisteban P (October 2007). "The forkhead factor FoxE1 binds to the thyroperoxidase promoter during thyroid cell differentiation and modifies compacted chromatin structure". Mol. Cell. Biol. 27 (20): 7302–14. doi:10.1128/MCB.00758-07. PMC 2168900
 . PMID 17709379.
. PMID 17709379. - 1 2 3 4 Zaret KS, Carroll JS (November 2011). "Pioneer transcription factors: establishing competence for gene expression". Genes Dev. 25 (21): 2227–41. doi:10.1101/gad.176826.111. PMC 3219227
 . PMID 22056668.
. PMID 22056668. - ↑ Xu CR, Cole PA, Meyers DJ, Kormish J, Dent S, Zaret KS (May 2011). "Chromatin "prepattern" and histone modifiers in a fate choice for liver and pancreas". Science. 332 (6032): 963–6. doi:10.1126/science.1202845. PMC 3128430
 . PMID 21596989.
. PMID 21596989. - ↑ Voss TC, Schiltz RL, Sung MH, et al. (August 2011). "Dynamic exchange at regulatory elements during chromatin remodeling underlies assisted loading mechanism". Cell. 146 (4): 544–54. doi:10.1016/j.cell.2011.07.006. PMC 3210475
 . PMID 21835447.
. PMID 21835447. - ↑ Liber D, Domaschenz R, Holmqvist PH, et al. (July 2010). "Epigenetic priming of a pre-B cell-specific enhancer through binding of Sox2 and Foxd3 at the ESC stage". Cell Stem Cell. 7 (1): 114–26. doi:10.1016/j.stem.2010.05.020. PMID 20621055.
- ↑ Katiyar P, Aplin AE (May 2011). "FOXD3 regulates migration properties and Rnd3 expression in melanoma cells". Mol. Cancer Res. 9 (5): 545–52. doi:10.1158/1541-7786.MCR-10-0454. PMC 3096755
 . PMID 21478267.
. PMID 21478267. - ↑ Heinz S, Benner C, Spann N, et al. (May 2010). "Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities". Mol. Cell. 38 (4): 576–89. doi:10.1016/j.molcel.2010.05.004. PMC 2898526
 . PMID 20513432.
. PMID 20513432. - ↑ Sérandour AA, Avner S, Percevault F, et al. (April 2011). "Epigenetic switch involved in activation of pioneer factor FOXA1-dependent enhancers". Genome Res. 21 (4): 555–65. doi:10.1101/gr.111534.110. PMC 3065703
 . PMID 21233399.
. PMID 21233399. - ↑ Taube JH, Allton K, Duncan SA, Shen L, Barton MC (May 2010). "Foxa1 functions as a pioneer transcription factor at transposable elements to activate Afp during differentiation of embryonic stem cells". J. Biol. Chem. 285 (21): 16135–44. doi:10.1074/jbc.M109.088096. PMC 2871482
 . PMID 20348100.
. PMID 20348100. - ↑ Smale ST (October 2010). "Pioneer factors in embryonic stem cells and differentiation". Curr. Opin. Genet. Dev. 20 (5): 519–26. doi:10.1016/j.gde.2010.06.010. PMC 2943026
 . PMID 20638836.
. PMID 20638836. - ↑ Xu J, Watts JA, Pope SD, et al. (December 2009). "Transcriptional competence and the active marking of tissue-specific enhancers by defined transcription factors in embryonic and induced pluripotent stem cells". Genes Dev. 23 (24): 2824–38. doi:10.1101/gad.1861209. PMC 2800090
 . PMID 20008934.
. PMID 20008934. - ↑ Chen SS, Raval A, Johnson AJ, et al. (August 2009). "Epigenetic changes during disease progression in a murine model of human chronic lymphocytic leukemia". Proc. Natl. Acad. Sci. U.S.A. 106 (32): 13433–8. doi:10.1073/pnas.0906455106. PMC 2726368
 . PMID 19666576.
. PMID 19666576. - 1 2 Caravaca JM, Donahue G, Becker JS, He X, Vinson C, Zaret KS (February 2013). "Bookmarking by specific and nonspecific binding of FoxA1 pioneer factor to mitotic chromosomes". Genes Dev. 27 (3): 251–60. doi:10.1101/gad.206458.112. PMID 23355396.
- ↑ Sekiya T, Zaret KS (October 2007). "Repression by Groucho/TLE/Grg proteins: genomic site recruitment generates compacted chromatin in vitro and impairs activator binding in vivo". Mol. Cell. 28 (2): 291–303. doi:10.1016/j.molcel.2007.10.002. PMC 2083644
 . PMID 17964267.
. PMID 17964267. - 1 2 3 4 5 6 Jozwik KM, Carroll JS (June 2012). "Pioneer factors in hormone-dependent cancers". Nat. Rev. Cancer. 12 (6): 381–5. doi:10.1038/nrc3263. PMID 22555282.
- ↑ Ross-Innes CS, Stark R, Teschendorff AE, et al. (January 2012). "Differential oestrogen receptor binding is associated with clinical outcome in breast cancer". Nature. 481 (7381): 389–93. doi:10.1038/nature10730. PMC 3272464
 . PMID 22217937.
. PMID 22217937. - ↑ Yang JY, Hung MC (February 2009). "A new fork for clinical application: targeting forkhead transcription factors in cancer". Clin. Cancer Res. 15 (3): 752–7. doi:10.1158/1078-0432.CCR-08-0124. PMC 2676228
 . PMID 19188143.
. PMID 19188143.