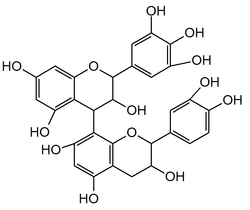
Prodelphinidin B3
 | |
| Names | |
|---|---|
| Other names | |
| Identifiers | |
| 78362-05-7 | |
| Properties | |
| C30H26O13 | |
| Molar mass | 594.52 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Prodelphinidin B3 is a prodelphinidin dimer found in food products such as barley[1][2] and beer, in fruits and pod vegetables. It can also be found in pomegranate peels.[3]
It can also be synthetized.[4]
References
- ↑ Klausen, K; Mortensen, AG; Laursen, B; Haselmann, KF; Jespersen, BM; Fomsgaard, IS (2010). "Phenolic compounds in different barley varieties: Identification by tandem mass spectrometry (QStar) and NMR; quantification by liquid chromatography triple quadrupole-linear ion trap mass spectrometry (Q-Trap)". Natural product communications. 5 (3): 407–14. PMID 20420318.
- ↑ Quinde-Axtell, Zory; Baik, Byung-Kee (2006). "Phenolic Compounds of Barley Grain and Their Implication in Food Product Discoloration". Journal of Agricultural and Food Chemistry. 54 (26): 9978–84. doi:10.1021/jf060974w. PMID 17177530.
- ↑ Plumb, G. W.; De Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J. C.; Williamson, G. (2002). "Antioxidant properties of gallocatechin and prodelphinidins from pomegranate peel". Redox Report. 7 (1): 41–6. doi:10.1179/135100002125000172. PMID 11981454.
- ↑ Delcour, Jan A.; Vercruysse, Sabine A. R. (1986). "Direct Synthesis of the Barley Proanthocyanidins Prodelphinidin B3, Prodelphinidin C2 and Two Trimeric Proanthocyanidins with a Mixed Prodelphinidin-Procyanidin Stereochemistry". Journal of the Institute of Brewing. 92 (3): 244. doi:10.1002/j.2050-0416.1986.tb04409.x.
External links
This article is issued from Wikipedia - version of the 7/7/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.