Proguanil
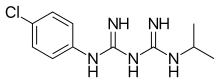 | |
| Clinical data | |
|---|---|
| Trade names | Paludrine |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | By mouth (tablets) |
| ATC code | P01BB01 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 75% |
| Metabolism | By liver (CYP2C19) |
| Metabolites | cycloguanil and 4-chlorophenylbiguanide |
| Biological half-life | 12–21 hours[1] |
| Identifiers | |
| |
| CAS Number |
500-92-5 |
| PubChem (CID) | 4923 |
| DrugBank |
DB01131 |
| ChemSpider |
4754 |
| UNII |
S61K3P7B2V |
| KEGG |
D08428 |
| ChEBI |
CHEBI:8455 |
| ChEMBL |
CHEMBL1377 |
| ECHA InfoCard | 100.007.196 |
| Chemical and physical data | |
| Formula | C11H16ClN5 |
| Molar mass | 253.731 g/mol |
| 3D model (Jmol) | Interactive image |
| Melting point | 129 °C (264 °F) |
| |
| |
| (verify) | |
Proguanil (also known as chlorguanide and chloroguanide) is an antimalarial medication. It is used with atovaquone for the treatment and prevention of Plasmodium falciparum that is resistant to chloroquine. It is also effective in the treatment of most other multi-drug resistant forms of P. falciparum and the success rate exceed 93%.[2]
Proguanil is a prodrug that is converted by the liver to its active metabolite, cycloguanil.[3][4]
It was the first antifolate developed for malaria, and reports of its efficacy date back to the 1940’s.[5] It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[6] In the United States and Canada, proguanil is only available in combination as atovaquone/proguanil.[7]
Medical uses
Proguanil is used for the prevention and treatment of malaria in both adults and children, particularly in areas where chloroquine-resistant P. faliciparum malaria has been reported. It is usually taken in combination with atovaquone, another antimalarial drug, in drug products such as Malarone or Malanil.[8]
Side effects
Proguanil is generally well-tolerated and most people do not experience side effects. However, common side effects include abdominal pain, nausea, headache, and fever. Taking proguanil with food may lessen these side effects.[9] Proguanil should not be taken by people with severe renal impairment, pregnant women, or women who are breastfeeding children less than 5 kg.[10] There have also been reports of increased levels of liver enzymes, which may remain high for up to 4 weeks after completion of treatment.[11]
Mechanism
When used alone, proguanil functions as a prodrug. It’s active metabolite, cycloguanil, is an inhibitor of dihydrofolate reductase (DHFR).[12] Both mammals and parasites have the enzyme DHFR. However, cycloguanil is specific for parasitic DHFR. This enzyme is a critical component of the folic acid cycle. Inhibition of DHFR prevents the parasite from recycling dihydrofolate back to tetrahydrofolate (THF.) THF acts as a metabolic narrows because it is required for all one carbon metabolism. Therefore, inhibition of this enzyme shuts down DNA synthesis, amino acid synthesis, and methylation.[13]
Proguanil displays synergism when used in combination with the antimalarial atovaquone. This mechanism of action differs from when proguanil was used as a singular agent. In this case, it is not thought to function as a DHFR inhibitor. The addition of proguanil has shown to reduce resistance to atovaquone and increase the ability of atovaquone to trigger a mitochondrial apoptotic cascade.[14] This is commonly referred to as “collapse of the mitochondrial membrane potential.”[15] Proguanil lowers the effective concentration of atovaquone needed to increase permeability of the mitochondrial membrane.[16]
References
- ↑ "Malarone (atovaquone/proguanil) Tablets, Pediatric Tablets. Full Prescribing Information" (PDF). GlaxoSmithKline. Research Triangle Park, NC 27709. Retrieved 14 July 2016.
- ↑ Boggild, Andrea K.; Parise, Monica E.; Lewis, Linda S.; Kain, Kevin C. (2007-02-01). "Atovaquone-Proguanil: Report from the Cdc Expert Meeting on Malaria Chemoprophylaxis (ii)". The American Journal of Tropical Medicine and Hygiene. 76 (2): 208–223. ISSN 0002-9637. PMID 17297027.
- ↑ Crowther, AF; Levi, AA (March 1953). "Proguanil—the Isolation of a Metabolite with High Antimalarial Activity". British Journal of Pharmacology and Chemotherapy. 8 (1): 93–7. doi:10.1111/j.1476-5381.1953.tb00758.x. PMC 1509229
 . PMID 13066702.
. PMID 13066702. - ↑ Pubchem. "proguanil | C11H16ClN5 - PubChem". pubchem.ncbi.nlm.nih.gov. Retrieved 2016-11-17.
- ↑ Nzila, Alexis (2006-06-01). "The past, present and future of antifolates in the treatment of Plasmodium falciparum infection". Journal of Antimicrobial Chemotherapy. 57 (6): 1043–1054. doi:10.1093/jac/dkl104. ISSN 0305-7453. PMID 16617066.
- ↑ "WHO Model List of Essential Medicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ "Proguanil". www.medscape.com. Medscape. Retrieved 8 November 2016.
- ↑ "Malaria: MedlinePlus Medical Encyclopedia". medlineplus.gov. Retrieved 2016-11-16.
- ↑ "Atovaquone And Proguanil (Oral Route) Side Effects - Mayo Clinic". www.mayoclinic.org. Retrieved 2016-11-08.
- ↑ Prevention, CDC - Centers for Disease Control and. "CDC - Malaria - Travelers - Choosing a Drug to Prevent Malaria". www.cdc.gov. Retrieved 2016-11-08.
- ↑ Looareesuwan, S.; Wilairatana, P.; Chalermarut, K.; Rattanapong, Y.; Canfield, C. J.; Hutchinson, D. B. (1999-04-01). "Efficacy and safety of atovaquone/proguanil compared with mefloquine for treatment of acute Plasmodium falciparum malaria in Thailand". The American Journal of Tropical Medicine and Hygiene. 60 (4): 526–532. ISSN 0002-9637. PMID 10348224.
- ↑ Pubchem. "proguanil | C11H16ClN5 - PubChem". pubchem.ncbi.nlm.nih.gov. Retrieved 2016-11-13.
- ↑ Boggild, Andrea K.; Parise, Monica E.; Lewis, Linda S.; Kain, Kevin C. (2007-02-01). "Atovaquone-Proguanil: Report from the Cdc Expert Meeting on Malaria Chemoprophylaxis (ii)". The American Journal of Tropical Medicine and Hygiene. 76 (2): 208–223. ISSN 0002-9637. PMID 17297027.
- ↑ Srivastava, Indresh K.; Vaidya, Akhil B. (1999-06-01). "A Mechanism for the Synergistic Antimalarial Action of Atovaquone and Proguanil". Antimicrobial Agents and Chemotherapy. 43 (6): 1334–1339. ISSN 0066-4804. PMC 89274
 . PMID 10348748.
. PMID 10348748. - ↑ Srivastava, Indresh K.; Rottenberg, Hagai; Vaidya, Akhil B. (1997-02-14). "Atovaquone, a Broad Spectrum Antiparasitic Drug, Collapses Mitochondrial Membrane Potential in a Malarial Parasite". Journal of Biological Chemistry. 272 (7): 3961–3966. doi:10.1074/jbc.272.7.3961. ISSN 0021-9258.
- ↑ Thapar, MM; Gupta, S; Spindler, C; Wernsdorfer, WH; Björkman, A (May 2003). "Pharmacodynamic Interactions Among Atovaquone, Proguanil and Cycloguanil against Plasmodium falciparum in vitro". Transactions of the Royal Society of Tropical Medicine and Hygiene. 97 (3): 331–7. doi:10.1016/S0035-9203(03)90162-3. PMID 15228254.