Propane (data page)
This page provides supplementary chemical data on Propane.
Structure and properties
| Structure and properties | |
|---|---|
| Dielectric constant | (fluid) 1.6 ε0 at 0 °C |
| Magnetic susceptibility | -40 |
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point | 85.47 K (-187.68 °C), 0.0001 Pa |
| Critical point | 369.522 K (96.672 °C), 42.4924 bar |
| Std enthalpy change of fusion, ΔfusH |
79.96 J/g |
| Std entropy change of fusion, ΔfusS |
? J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
24.545 kJ/mol |
| Std entropy change of vaporization, ΔvapS |
? J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
-103.85[1] kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
−118.910 kJ/mol |
| Standard molar entropy, S |
171.0 J/(mol K) |
| Heat capacity, cp | 98.36 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
−104.7 kJ/mol |
| Standard molar entropy, S |
269.91 J/(mol K) |
| Enthalpy of combustion, ΔcH |
–2202.0 kJ/mol |
| Heat capacity, cp | 73.60 J/(mol K) |
| van der Waals' constants[2] | a = 877.88 L2 kPa/mol2 b = 0.08445 liter per mole |
Vapor pressure of liquid
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | 1520 | 3800 | 7600 | 15200 | 30400 | 45600 | |
| T in °C | –128.9 | –108.5 | –92.4 | –79.6 | –55.6 | –42.1 | –25.6 | 1.4 | 25.6 | 58.1 | 94.8 | — | |
Table data obtained from CRC Handbook of Chemistry and Physics 44th ed.
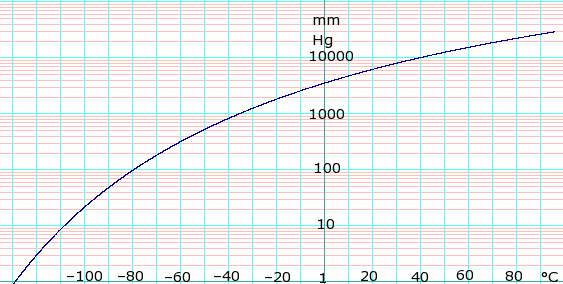
log of propane vapor pressure. Uses formula: from Lange's Handbook of Chemistry, 10th ed.
Spectral data
| UV-Vis | |
|---|---|
| λmax | ? nm |
| Extinction coefficient, ε | ? |
| IR | |
| Major absorption bands | ? cm−1 |
| NMR | |
| Proton NMR | |
| Carbon-13 NMR | |
| Other NMR data | |
| MS | |
| Masses of main fragments |
|
Material Safety Data Sheet
Propane does not have health effects other than the danger of frostbite or asphyxiaton. The National Propane Gas Association has a generic MSDS available online here. (Issued 1996)
- MSDS from Suburban Propane, L.P dated 5/2013 in the SDSdata.org database
Except where noted otherwise, data relate to standard ambient temperature and pressure.
References
External links
Disclaimer applies.
This article is issued from Wikipedia - version of the 9/6/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.