Particle statistics
| Statistical mechanics |
|---|
 |
Particle statistics is a particular description of multiple particles in statistical mechanics. Its core concept is a statistical ensemble that emphasizes properties of a large system as a whole at the expense of knowledge about parameters of separate particles. When an ensemble consists of particles with similar properties, their number is called the particle number and usually denoted by N.
Classical statistics
In classical mechanics all the particles (fundamental and composite particles, atoms, molecules, electrons, etc.) in the system are considered distinguishable. This means that one can label and track each individual particle in a system. As a consequence, changing the position of any two particles in the system leads to a completely different configuration of the entire system. Furthermore, there is no restriction on placing more than one particle in any given state accessible to the system. Classical statistics is called Maxwell–Boltzmann statistics (or M–B statistics).
Quantum statistics
The fundamental feature of quantum mechanics that distinguishes it from classical mechanics is that particles of a particular type are indistinguishable from one another. This means that in an assembly consisting of similar particles, interchanging any two particles does not lead to a new configuration of the system (in the language of quantum mechanics: the wave function of the system is invariant up to a phase with respect to the interchange of the constituent particles). In the case of a system consisting of particles of different kinds (for example, electrons and protons), the wave function of the system is invariant up to a phase separately for both assemblies of particles.
The applicable definition of a particle does not require it to be elementary or even "microscopic", but it requires that all its degrees of freedom (or internal states) that are relevant to the physical problem considered shall be known. All quantum particles, such as leptons and baryons, in the universe have three translational motion degrees of freedom (represented with the wave function) and one discrete degree of freedom, known as spin. Progressively more "complex" particles obtain progressively more internal freedoms (such as various quantum numbers in an atom), and when the number of internal states, that "identical" particles in an ensemble can occupy, dwarfs their count (the particle number), then effects of quantum statistics become negligible. That's why quantum statistics is useful when one considers, say, helium liquid or ammonia gas (its molecules have a large, but conceivable number of internal states), but is useless applied to systems constructed of macromolecules.
While this difference between classical and quantum descriptions of systems is fundamental to all of quantum statistics, quantum particles are divided into two further classes on the basis of the symmetry of the system. The spin–statistics theorem binds two particular kinds of combinatorial symmetry with two particular kinds of spin symmetry, namely bosons and fermions.
Bose–Einstein statistics
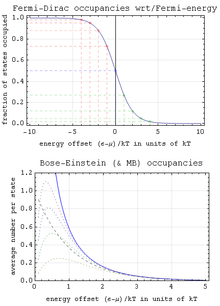
In Bose–Einstein statistics (B–E Statistics) interchanging any two particles of the system leaves the resultant system in a symmetric state. That is, the wave function of the system before interchanging equals the wave function of the system after interchanging.
It is important to emphasize that the wave function of the system has not changed itself. This has very important consequences on the state of the system: There is no restriction to the number of particles that can be placed in a single state (accessible to the system). It is found that the particles that obey Bose–Einstein statistics are the ones which have integer spins, which are therefore called bosons (named after Bose). Examples of bosons include photons and helium-4 (both atoms and nuclei). One type of system obeying B–E statistics is the Bose–Einstein condensate where all particles of the assembly exist in the same state.
Fermi–Dirac statistics

In Fermi–Dirac statistics (F–D statistics) interchanging any two particles of the system leaves the resultant system in an antisymmetric state. That is, the wave function of the system before interchanging is the wave function of the system after interchanging, with an overall minus sign.
Again, the wave function of the system itself does not change. The consequence of the negative sign on the Fermi–Dirac statistics can be understood in the following way:
Suppose that the particles that are interchanged belong to the same state. Since the particles are considered indistinguishable from one another then changing the coordinates of the particles should not have any change on the system's wave function (because by our assumptions the particles are in the same state). Therefore, the wave function before interchanging similar states equals the wave function after interchanging similar states.
Combining (or adding, literally speaking) the above statement with the fundamental asymmetry of the Fermi–Dirac system leads us to conclude that the wave function of the system before interchanging equals zero.
This shows that in Fermi–Dirac statistics, more than one particle cannot occupy a single state accessible to the system. This is called Pauli's exclusion principle.
It is found that particles with half-integral spin (or fermions) obey the Fermi–Dirac statistics. This includes electrons, protons, helium-3 (both atoms and nuclei) etc.