Resiniferatoxin
 | |
| Names | |
|---|---|
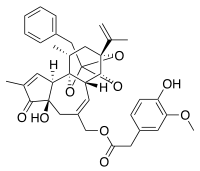
| IUPAC name
[(1R,6R,13R,15R,17R)-13-benzyl-6-hydroxy-4,17-dimethyl-5-oxo-15-(prop-1-en-2-yl)-12,14,18-trioxapentacyclo[11.4.1.0¹,¹⁰.0²,⁶.0¹¹,¹⁵]octadeca-3,8-dien-8-yl]methyl 2-(4-hydroxy-3-methoxyphenyl)acetate | |
| Identifiers | |
| 57444-62-9 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL448382 |
| ChemSpider | 21106474 |
| ECHA InfoCard | 100.165.067 |
| 2491 | |
| MeSH | resiniferatoxin |
| PubChem | 104826 |
| |
| |
| Properties | |
| C37H40O9 | |
| Molar mass | 628.71 g/mol |
| Density | 1.35 ± 0.1 g/cm³ |
| insoluble | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Resiniferatoxin (RTX) is a naturally occurring chemical found in resin spurge (Euphorbia resinifera), a cactus-like plant commonly found in Morocco, and in Euphorbia poissonii found in northern Nigeria.[1] It is an ultrapotent analog of capsaicin, the active ingredient in chili peppers.[2]
Overview
Resiniferatoxin activates transient vanilloid receptor 1 (TRPV1) in a subpopulation of primary afferent sensory neurons involved in nociception (the transmission of physiological pain).[3][4] TRPV1 is an ion channel in the plasma membrane of sensory neurons and stimulation by resiniferatoxin causes this ion channel to become permeable to cations, especially calcium. The influx of cations causes the neuron to depolarize, transmitting signals similar to those that would be transmitted if the innervated tissue were being burned or damaged. This stimulation is followed by desensitization and analgesia, in part because the nerve endings die from calcium overload.[5][6]
Total synthesis
A total synthesis of (+)-resiniferatoxin was completed by the Wender group at Stanford University in 1997.[7] As of 2007, this represented the only complete total synthesis of any member of the daphnane family of molecules.[8]
Toxicity
Resiniferatoxin is toxic and can inflict a chemical burning sensation in microscopic quantities. The primary action of resiniferatoxin is to activate sensory neurons responsible for the perception of pain. It is currently the most potent TRPV1 agonist known to science, with ~500x higher binding affinity for TRPV1 than capsaicin, the active ingredient in hot chili peppers such as those produced by Capsicum annuum. Animal experiments suggest that, in humans, ingestion of 10 g may be fatal or cause serious damage to health.[9] It causes severe burning pain in sub-microgram (less than 1/1,000,000th of a gram) quantities when ingested orally.
See also
- Discovery and development of TRPV1 antagonists
- Iodoresiniferatoxin
- Transient receptor potential
- Tinyatoxin
References
- ↑ Euphorbia poissonii in BoDD – Botanical Dermatology Database
- ↑
- Christopher S. J. Walpole; et al. (1996). "Similarities and Differences in the Structure-Activity Relationships of Capsaicin and Resiniferatoxin Analogues". J. Med. Chem. 39 (15): 2939–2952. doi:10.1021/jm960139d. PMID 8709128.
- ↑ Szallasi A, Blumberg PM (1989). "Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper". Neuroscience. 30 (2): 515–520. doi:10.1016/0306-4522(89)90269-8. PMID 2747924.
- ↑ Szallasi A, Blumberg PM (1990). "Resiniferatoxin and its analogs provide novel insights into the pharmacology of the vanilloid (capsaicin) receptor". Life Sci. 47 (16): 1399–1408. doi:10.1016/0024-3205(90)90518-V.
- ↑ Szallasi A, Blumberg PM (1992). "Vanilloid receptor loss in rat sensory ganglia associated with long term desensitization to resiniferatoxin". Neurosci Lett. 140 (1): 51–54. doi:10.1016/0304-3940(92)90679-2. PMID 1407700.
- ↑ Olah Z, et al. (2001). "Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1". J. Biol. Chem. 276 (14): 11021–11030. doi:10.1074/jbc.M008392200. PMID 11124944.
- ↑ Wender, P.A.; Jesudason, Cynthia D.; Nakahira, Hiroyuki; Tamura, Norikazu; Tebbe, Anne Louise; Ueno, Yoshihide (1997). "The First Synthesis of a Daphnane Diterpene: The Enantiocontrolled Total Synthesis of (+)-Resiniferatoxin". J. Am. Chem. Soc. 119 (52): 12976–12977. doi:10.1021/ja972279y.
- ↑ http://www.scripps.edu/chem/baran/images/grpmtgpdf/Seiple_Mar_07.pdf
- ↑ Material Safety Data Sheet for resiniferatoxin, 2009