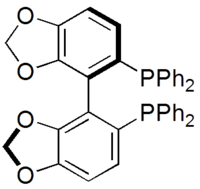
SEGPHOS
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4,4'-Bi-1,3-benzodioxole-5,5'-diylbis(diphenylphosphane) | |
| Other names
SEGPHOS 5,5′-Bis(diphenylphosphino)-4,4′-bi-1,3-benzodioxole | |
| Identifiers | |
| 244261-66-3 (R) 210169-54-3 (S) | |
| Properties | |
| C38H28O4P2 | |
| Molar mass | 610.57 g/mol |
| Appearance | colorless solid |
| organic solvents | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
SEGPHOS is a chiral ligand developed by Takasago that is used in asymmetric synthesis.[1] It was developed after BINAP and was investigated since it has a narrower dihedral angle between the aromatic faces. This was predicted and then confirmed to increase the enantioselectivity and activity of metal complexes of SEGPHOS.
In addition to the parent ligand bearing phenyl groups on the phosphorus atoms, the bulkier derivatives DM-SEGPHOS and DTBM-SEGPHOS are also commercially available.[2] In DM-SEGPHOS and DTBM-SEGPHOS, the phenyl groups of SEGPHOS are replaced by 3,5-dimethylphenyl and 3,5-di-t-butyl-4-methoxyphenyl groups, respectively.

References
- ↑ Shimizu, H., Nagasaki, I., Matsumura, K., Sayo, N., Saito, T. (2007). "Developments in Asymmetric Hydrogenation from an Industrial Perspective". Acc. Chem. Res. 40 (12): 1385–1393. doi:10.1021/ar700101x. PMID 17685581.
- ↑ "SEGPHOS". www.strem.com. Retrieved 2016-08-10.
This article is issued from Wikipedia - version of the 8/10/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.