Saline water
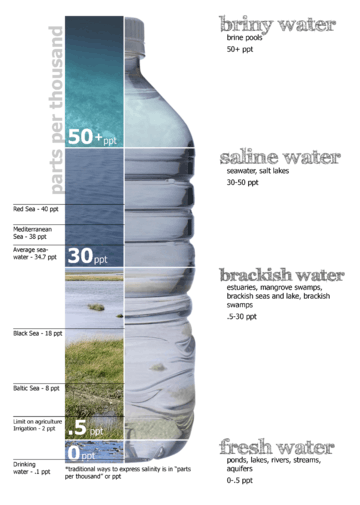
Saline water (more commonly known as salt water) is water that contains a significant concentration of dissolved salts (mainly NaCl). The salt concentration is usually expressed in parts per thousand (permille, ‰) or parts per million (ppm). The United States Geological Survey classifies saline water in three salinity categories. Salt concentration in slightly saline water is around 1,000 to 3,000 ppm (0.1–0.3%), in moderately saline water 3,000 to 10,000 ppm (0.3–1%) and in highly saline water 10,000 to 35,000 ppm (1–3.5%). Seawater has a salinity of roughly 35,000 ppm, equivalent to 35 grams of salt per one liter (or kilogram) of water. The saturation level is dependent on the temperature of the water. At 20 °C one milliliter of water can dissolve about 0.357 grams of salt; a concentration of 26.3%. At boiling (100 °C) the amount that can be dissolved in one milliliter of water increases to about 0.391 grams or 28.1% saline solution.[1]
Some industries make use of saline water, such as mining and thermo-electric power.
| Fresh water | Brackish water | Saline water | Brine |
|---|---|---|---|
| < 0.05% | 0.05–3% | 3–5% | > 5% |
Use in the United States
In the United States, 14 percent of all water used in 2000 was saline.[2] Almost all saline withdrawals, over 92 percent, were used by the thermo-electric power industry to cool electricity-generating equipment. About three percent of the nation's saline water was used for mining and other industrial purposes.[2]
Due to their proximity to the Atlantic and Pacific Oceans, states near the coast make the most use of saline water. Almost 40% of all saline water use in 2000 occurred in California, Florida, and Maryland.[2]
The use of saline water, as with freshwater, has been trending downward since a peak in 1968. But, in the period between 1950 and 1968, the use of saline water increased at a much higher rate than freshwater use.[2]
Properties
The thermal conductivity of seawater is 0.6 W/mK at 25 °C and a salinity of 35 g/kg.. The thermal conductivity decreases with increasing salinity and increases with increasing temperature; these graphs and online calculations plot thermal conductivity for varying salinity and temperature: The salt content can be determined with a salinometer.
See also
References
- ↑ "Solubility". Fundamentals of Chemistry. 2000. Retrieved 6 November 2014.
- 1 2 3 4 Uses of saline water, USGS
External links
![]() Media related to Saline water at Wikimedia Commons
Media related to Saline water at Wikimedia Commons