Arsphenamine
Arsphenamine, also known as Salvarsan or compound 606, is a drug that was introduced at the beginning of the 1910s as the first effective treatment for syphilis, and was also used to treat trypanosomiasis.[2] This organoarsenic compound was the first modern chemotherapeutic agent.
History
Arsphenamine was first synthesized in 1907 in Paul Ehrlich's lab by Alfred Bertheim.[3] The antisyphilitic activity of this compound was discovered by Sahachiro Hata in 1909, during a survey of hundreds of newly synthesized organic arsenical compounds. Ehrlich had theorized that by screening many compounds, a drug could be discovered that would have anti-microbial activity but not kill the human patient. Ehrlich's team began their search for such a "magic bullet" among chemical derivatives of the dangerously toxic drug atoxyl. This project was the first organized team effort to optimize the biological activity of a lead compound through systematic chemical modifications, the basis for nearly all modern pharmaceutical research.
Arsphenamine was used to treat the disease syphilis because it is toxic to the bacterium Treponema pallidum, a spirochete that causes syphilis.
Arsphenamine was originally called "606" because it was the sixth in the sixth group of compounds synthesized for testing; it was marketed by Hoechst AG under the trade name Salvarsan in 1910.[4][5] Salvarsan was the first organic antisyphilitic, and a great improvement over the inorganic mercury compounds that had been used previously. It was distributed as a yellow, crystalline, hygroscopic powder that was highly unstable in air.[6] This significantly complicated administration, as the drug had to be dissolved in several hundred milliliters of distilled, sterile water with minimal exposure to air to produce a solution suitable for injection. Some of the side effects attributed to Salvarsan, including rashes, liver damage, and risks of life and limb, were thought to be caused by improper handling and administration.[7] This caused Ehrlich, who worked assiduously to standardize practices, to observe, "the step from the laboratory to the patient's bedside ... is extraordinarily arduous and fraught with danger." [4]
Ehrlich's laboratory developed a more soluble (but slightly less effective) arsenical compound, Neosalvarsan (neoarsphenamine), which was easier to prepare, and it became available in 1912. Less severe side-effects such as nausea and vomiting were still common. An additional problem was that both Salvarsan and Neosalvarsan had to be stored in sealed vials under a nitrogen atmosphere to prevent oxidation. These arsenical compounds were supplanted as treatments for syphilis in the 1940s by penicillin.[8]
After leaving Ehrlich's laboratory, Hata continued parallel investigation of the new medicines in Japan.[9]
Structure
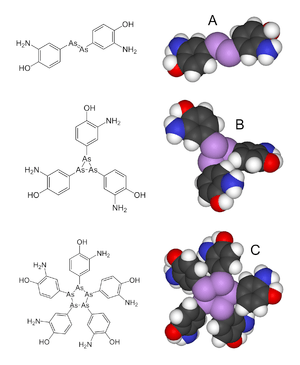
Salvarsan has long been assumed to have an As=As double bond, akin to the N=N linkage in azobenzene. However, in 2005, an extensive mass spectral analysis Salvarsan was shown to have As-As single bonds, not As=As double bonds. The drug was also found to be a mixture consisting of cyclo-As3 and cyclo-As5 species.[1][4][10]
See also
- Dr. Ehrlich's Magic Bullet, 1940 film about Ehrlich's quest to find a cure for syphilis.
References
- 1 2 Lloyd NC, Morgan HW, Nicholson BK, Ronimus RS (2005). "The composition of Ehrlich's salvarsan: resolution of a century-old debate". Angew. Chem. Int. Ed. Engl. 44 (6): 941–4. doi:10.1002/anie.200461471. PMID 15624113.
- ↑ Gibaud, Stéphane; Jaouen, Gérard (2010). "Arsenic - based drugs: from Fowler's solution to modern anticancer chemotherapy". Topics in Organometallic Chemistry. Topics in Organometallic Chemistry. 32: 1–20. doi:10.1007/978-3-642-13185-1_1. ISBN 978-3-642-13184-4.
- ↑ Williams KJ (2009). "The introduction of 'chemotherapy' using arsphenamine - the first magic bullet". J R Soc Med. 102: 343–8. doi:10.1258/jrsm.2009.09k036. PMC 2726818
 . PMID 19679737.
. PMID 19679737. - 1 2 3 "Salvarsan". Chemical & Engineering News. Retrieved 2010-02-01.
- ↑ In Germany, it was the practice to designate compounds by their development number. Another compound known commonly in Germany by its number is parathion, which was the 605th compound to be developed in a search for insecticides. It is commonly known as E605 (E stands for Entwicklungsnummer, German for "development number").
- ↑ "A Handbook of Useful Drugs". American Medical Association. 1913. Retrieved 2010-08-17.
- ↑ http://archive.protomag.com/assets/paul-ehrlich-and-the-salvarsan-wars
- ↑ Bosch F, Rosich L (2008). "The contributions of Paul Ehrlich to pharmacology: a tribute on the occasion of the centenary of his Nobel Prize". Pharmacology. 82: 171–9. doi:10.1159/000149583. PMC 2790789
 . PMID 18679046.
. PMID 18679046. - ↑ Izumi, Yoshio; Isozumi, Kazuo (2001). "Modern Japanese medical history and the European influence" (free download pdf). Keio Journal of Medicine. 50 (2): 91–99. doi:10.2302/kjm.50.91. PMID 11450598.
- ↑ http://www.rsc.org/chemistryworld/2013/04/salvarsan-podcast