Secosteroid
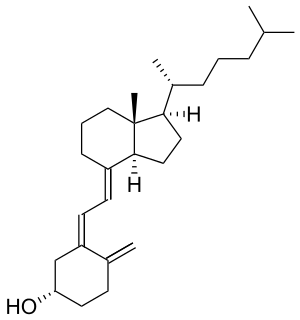
Cholecalciferol (vitamin D3), an example of a 9,10-secosteroid. The hydroxyl group (HO-) is in position C3 of the parent steroid A-ring. The triene substructure attached to the ring bearing the hydroxyl group is a result of the ring scission (cleavage) giving rise to this secosteroid.
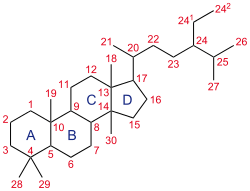
The parent steroid skeleton. The B-ring of the parent steroid is broken between C9 and C10 to yield the vitamins D.
A secosteroid (sec·o·ster·oid, sek'ō-stēr'oyd) is a type of steroid with a "broken" ring. The word secosteroid derives from the verb Latin: secare meaning "to cut",[1]:241 and Latin: stere of steroid, meaning "solid, three-dimensional".[1]:129 Secosteroids are alternatively described as a subclass of steroids[2] or derived from steroids.[3]
Types or subclasses of secosteroids are defined by the carbon atoms of the parent steroid skeleton where the ring cleavage has taken place. For example, 9,10-secosteroids derived from cleavage of the bond between carbon atoms C9 and C10 of the steroid B-ring (similarly 5,6-secosteroids, 13,14-steroids, etc.).
The prototypical secosteroid is cholecalciferol (vitamin D3).[4]
References
- 1 2 Ayers D (1972). Bioscientific Terminology. Tucson: University of Arizona Press. ISBN 978-0-8165-0305-6.
- ↑ Moss GP and the Working Party of the IUPAC-IUB Joint Commission on Biochemical Nomenclature. "The Nomenclature of Steroids". Queen Mary University of London. p. Section 3S-1 (esp. 3S-1.4, incl. note 4).; Moss GP (1989). "Nomenclature of Steroids (Recommendations 1989)". Pure & Appl. Chem. 61 (10): 1786f. doi:10.1351/pac198961101783.
Steroids are compounds possessing the skeleton of cyclopenta[a]phenanthrene or a skeleton derived therefrom by one or more bond scissions or ring expansions or contractions.
; Hill RA; Makin HL; Kirk DN; Murphy GM (1991). Dictionary of Steroids (1st ed.). London: Chapman & Hall. ISBN 978-0-412-27060-4. - ↑ "Definition of secosteroid". Farlex Partner Medical Dictionary. TheFreeDictionary.com.
A compound derived from a steroid in which there has been a ring cleavage.
- ↑ Hanson JR (2010). "Steroids: partial synthesis in medicinal chemistry". Nat Prod Rep. 27 (6): 887–99. doi:10.1039/c001262a. PMID 20424788.
External links
- Secosteroids at the US National Library of Medicine Medical Subject Headings (MeSH)
This article is issued from Wikipedia - version of the 11/20/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.