Sulfilimine
A sulfilimine (sulfimide) is a type of chemical compound containing a sulfur to nitrogen double bond. The parent compound is sulfilimine H2S=NH, which is mainly of theoretical interest. Other simple examples are methylphenylsulfoximine[2] and S,S-diphenylsulfilimine:[3]
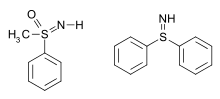 Methyl-phenylsulfoximine, a sulfur(VI) compound, and S,S-diphenylsulfilimine, a sulfur(IV) compound.
Methyl-phenylsulfoximine, a sulfur(VI) compound, and S,S-diphenylsulfilimine, a sulfur(IV) compound.
Preparation
Most sulfilimines are N-substituted with electron-withdrawing groups. These compounds are typically prepared by oxidation of thioethers with electrophilic amine reagents, such as chloramine-T in the presence of a base:[4]
- R2S + ClNHTs → R2S=NTs + HCl
An alternative route involves reactions of electrophilic sulfur compounds with amines. The limidosulfonium reagents provide a source of "Me2S2+", which are attacked by amines.
Sulfilimine bonds in proteins
Sulfilimine bonds stabilize collagen IV strands found in the extracellular matrix.[5] These bonds covalently connect hydroxylysine and methionine residues of adjacent polypeptide strands to form a larger collagen trimer.
References
- ↑ The preparation and structure of novel sulfimide systems; X-ray crystal structures of 1,4-(PhS{NH})2C6H4(and dihydrate), 1,2-(PhS{NH})(PhS)C6H4·H2O and of [Ph2SNH] and its hydrate Mark R. J. Elsegood, Kathryn E. Holmes, Paul F. Kelly, Jonathan Parr and Julia M. Stonehouse New J. Chem., 2002, 26, 202 - 206. doi:10.1039/b103502a
- ↑ sigmaaldrich.com/catalog methyl-phenylsulfoximine
- ↑ sigmaaldrich.com/catalog S,S-diphenylsulfilimine
- ↑ Gilchrist, T. L.; Moody, C. J., "The chemistry of sulfilimines", Chem. Rev. 1977, 77, 409-435. doi:10.1021/cr60307a005
- ↑ Vanacore R, Ham AL, Voehler M, Sanders CR, Conrads TP, Veenstra TD, Sharpless KB, Dawson PE, Hudson BG (September 4, 2009). "A sulfilimine bond identified in collagen IV.". Science. 325 (5945): 1230–1234. Bibcode:2009Sci...325.1230V. doi:10.1126/science.1176811. PMC 2876822
 . PMID 19729652.
. PMID 19729652.