Taeniasis
| Taeniasis | |
|---|---|
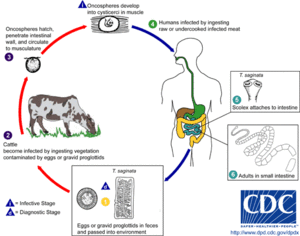 | |
| The life cycle of Taenia saginata, the beef tapeworm | |
| Classification and external resources | |
| Specialty | infectious disease |
| ICD-10 | B68 |
| ICD-9-CM | 123.3 |
| DiseasesDB | 12875 |
| MedlinePlus | 001391 |
| eMedicine | ped/2201 |
| MeSH | D013622 |
Taeniasis is a parasitic disease due to infection with tapeworms belonging to the genus Taenia. The two most important human pathogens in the genus are Taenia solium (the pork tapeworm) and Taenia saginata (the beef tapeworm). The third species Taenia asiatica is found only in East Asia. Taeniasis is generally asymptomatic, but severe infection causes weight loss, dizziness, abdominal pain, diarrhea, headaches, nausea, constipation, chronic indigestion, and loss of appetite.
A type of taeniasis called cysticercosis is caused by accidental infection with the eggs of T. solium from contaminated food and water. It is known as the most pathogenic form caused by tapeworms.[1] A specific form of cysticercosis called neurocysticercosis is said to be the most common infection of the central nervous system.
Signs and symptoms
Taeniasis is generally asymptomatic and is diagnosed when a portion of the worm is passed in the stool. It is not fatal, although cysticercosis can cause epilepsy and neurocysticercosis can be fatal.[2][3][4]
Taenia solium
Infection by T. solium is normally asymptomatic. Heavy infection is indicated by intestinal irritation, anaemia, and indigestion.
Cysticercosis
There are accidental consumptions of eggs of T. solium from contaminated vegetables or water. The eggs enter the intestine where they develop into larvae. The larvae enter bloodstream and invade host tissues. This clinical condition, called cysticercosis, is the most frequent and severe disease caused by any tapeworm. It can lead to severe headaches, dizziness, occasional seizures, dementia, hypertension, lesions in the brain, blindness, tumor-like growths, and low eosinophil levels. It is the cause of major neurological problems, such as hydrocephalus, paraplegy, meningitis, convulsions, and even death.[5]
Taenia saginata
Taenia saginata infection is asymptomatic, but heavy infection causes weight loss, dizziness, abdominal pain, diarrhea, headaches, nausea, constipation, chronic indigestion, and loss of appetite. It can cause antigen reaction that induce allergic reaction.[6] It is an also rare cause of ileus, pancreatitis, cholecystitis, and cholangitis.[7]
Taenia asiatica
Taenia asiatica is also usually asymptomatic. The only severe case was in a 60-year-old woman at the Mackay Memorial Hospital in Taiwan. Her stomach and intestine were severely damaged with active bleeding from ulcers caused by a single tapeworm.[8][9]
In pigs, the cysticercus can produce cysticercosis. Cysts develop in liver and lungs. (T. saginata does not cause cysticercosis.)[10] Due to its biological similarity to T. solium, which is the major cause of neurocysticercosis, T. asiatica may also cause cysticercosis.[11][12][13]
Transmission
Taeniasis is contracted after eating undercooked or raw pork and beef that contain the larvae. Cysticercosis occurs after ingestion of contaminated food, water, or soil that contain T. solium eggs.[14][15] Infection is acquired by eating undercooked beef and pork that contain the fluid-filled cysticerci of the tapeworms. The adult worms live in the lumen of the intestine where they cause the symptoms. They acquire nutrients from the intestine, leading to malnutrition of the host. The gravid proglottids, body segments containing fertilised eggs, are released in the faeces of the host. Then they are consumed by an intermediate host such as a cow or pig, where they hatch within the duodenum to become larvae, penetrate through the intestinal wall into nearby blood vessels, and enter the bloodstream. Once they reach organs such as the skeletal muscles, liver or lungs, the larvae then develop into a cyst, which then becomes a fluid-filled cysticercus. These contaminated tissues are then consumed through raw or undercooked meat.[2]
Diagnosis
Diagnosis of taeniasis is mainly using stool sample, particularly by identifying the eggs. However, this has limitation at the species level because tapeworms basically have similar eggs. Examination of the scolex or the gravid proglottids can resolve the exact species.[16] But body segments are not often available, therefore, laborious histological observation of the uterine branches and PCR detection of ribosomal 5.8S gene are sometimes necessary.[17][18] Ziehl–Neelsen stain is also used for T. saginata and T. solium, in most cases only the former will stain, but the method is not entirely reliable.[19] Loop-mediated isothermal amplification (LAMP) is highly sensitive (~2.5 times that of multiplex PCR), without false positives, for differentiating the taenid species from faecal samples.[20]
To date the most relevant test for T. asiatica is by enzyme-linked immunoelectrotransfer blot (EITB). EITB can effectively identify asiatica from other taenid infections since the serological test indicates an immunoblot band of 21.5 kDa exhibited specifically by T. asiatica.[21] Even though it gives 100% sensitivity, it has not been tested with human sera for cross-reactivity, and it may show a high false positive result.
Prevention
The fundamental prevention strategy is hygiene and sanitation. Secondary measures include stricter meat-inspection standards, livestock confinement, health education, safe meat preparation, mass drug therapy, and identifying and treating human and pig carriers.[22] Moreover, a high level of sanitation and prevention of human faecal contamination of pig feeds also plays a major role in prevention. Infection can be prevented with proper disposal of human faeces around pigs, cooking meat thoroughly and/or freezing the meat at −10 °C for 5 days. For human cysticercosis, dirty hands are attributed to be the primary cause, and especially common among food handlers.[2]
Proper cooking of meat is an effective prevention. For example, cooking (56 °C for 5 minutes) of beef viscera destroys cysticerci. Refrigeration, freezing (-10 °C for 9 days) or long periods of salting is also lethal to cysticerci. Inspection of beef and proper disposal of human excreta are also important measures.[6]
Treatment
Oral anti-parasitic drugs such as praziquantel are the treatment of choice. Treatment with praziquantel has been approved by the U.S. Food and Drug Administration and is quite effective against these parasites.[23] Usual treatments are with praziquantel (5–10 mg/kg, single-administration) or niclosamide (adults and children over 6 years: 2 g, single-administration after a light breakfast, followed after 2 hours by a laxative; children aged 2–6 years: 1 g; children under 2 years: 500 mg).[6] Albendazole is also highly effective.[24] Atrabine is quite effective but has adverse effects in humans.[25]
Epidemiology
Regions
Taeniasis is predominantly found in Asia, Africa, Latin America, particularly on farms in which pigs are exposed to human excrement. It occurs everywhere though where beef and pork are eaten, even in countries such as the United States, with strict federal sanitation policies. Taenia saginata is relatively common in Africa, some parts of Eastern Europe, the Philippines, and Latin America.[26] It is most prevalent in Sub-Saharan Africa and the Middle East.[27] Taenia asiatica is retricted to East Asia, including Taiwan, Korea, Indonesia, Nepal, Thailand and China.[28][29]
Infection estimates
The total global infection is estimated to be between 40 and 60 million people.[30] In the US, the incidence of infection is low, but 25% of cattle sold are still infected.[16]
See also
References
- ↑ "Neglected Tropical Diseases". cdc.gov. June 6, 2011. Retrieved 28 November 2014.
- 1 2 3 Garcia, Oscar H. Del Brutto, Hector H. (2014). "Taenia solium: Biological Characteristics and Life Cycle". Cysticercosis of the Human Nervous System. (1., 2014 ed.). Berlin: Springer-Verlag Berlin and Heidelberg GmbH & Co. KG. pp. 11–21. ISBN 978-3-642-39021-0.
- ↑ "About Taeniasis/cysticercosis". Retrieved 13 March 2014.
- ↑ "Signs, symptoms and treatment of taeniasis/cysticercosis". Retrieved 13 March 2014.
- ↑ Flisser, A.; Avila G; Maravilla P; Mendlovic F; León-Cabrera S; Cruz-Rivera M; Garza A; Gómez B; Aguilar L; Terán N; Velasco S; Benítez M; Jimenez-Gonzalez DE (2010). "Taenia solium: current understanding of laboratory animal models of taeniosis". Parasitology. 137 (03): 347–57. doi:10.1017/S0031182010000272. PMID 20188011.
- 1 2 3 "Taeniasis/Cysticercosis". WHO Fact sheet N°376. World Health Organization. 2013. Retrieved 7 February 2014.
- ↑ Uygur-Bayramiçli, O; Ak, O; Dabak, R; Demirhan, G; Ozer, S (2012). "Taenia saginata a rare cause of acute cholangitis: a case report". Acta Clinica Belgica. 67 (6): 436–7. doi:10.1179/ACB.67.6.2062709. PMID 23340150.
- ↑ Liao, Wen-Shen; Bair, Ming Jong (2007). "Taenia in the Gastrointestinal Tract". New England Journal of Medicine. 357 (10): 1028–1028. doi:10.1056/NEJMicm067761. PMID 17804847.
- ↑ McManus, Donald P. (2008). "Taenia in the Gastrointestinal Tract". New England Journal of Medicine. 358 (3): 311–311. doi:10.1056/NEJMc072882. PMID 18199875.
- ↑ Galán-Puchades, M.T.; Fuentes, M.V. (2008). "Taenia asiatica and pig cysticercosis". Veterinary Parasitology. 157 (1-2): 160–161. doi:10.1016/j.vetpar.2008.07.008. PMID 18752896.
- ↑ Galán-Puchades, M. Teresa; Fuentes, Mario V. (2013). "Taenia asiatica : the most neglected human Taenia and the possibility of cysticercosis". The Korean Journal of Parasitology. 51 (1): 51–4. doi:10.3347/kjp.2013.51.1.51. PMC 3587749
 . PMID 23467406.
. PMID 23467406. - ↑ Galán-Puchades, MT; Fuentes, MV (2013). "Lights and shadows of the Taenia asiatica life cycle and pathogenicity". Tropical Parasitology. 3 (2): 114–9. doi:10.4103/2229-5070.122114. PMC 3889087
 . PMID 24470994.
. PMID 24470994. - ↑ Galán-Puchades, M. T.; Fuentes, M. V. (2009). "Diagnosis of Human Cysticercosis and Taenia asiatica". American Journal of Tropical Medicine and Hygiene. 81 (6): 1165–1165. doi:10.4269/ajtmh.2009.09-0398a.
- ↑ Roberts, Larry S.; Janovy, Jr., John (2009). Gerald D. Schmidt & Larry S. Roberts' Foundations of Parasitology (8 ed.). Boston: McGraw-Hill Higher Education. pp. 348–351. ISBN 978-0-07-302827-9.
- ↑ "Transmission of taeniasis/cysticercosis". Retrieved 13 March 2014.
- 1 2 Jr, Larry S. Roberts, John Janovy, (2009). Gerald D. Schmidt & Larry S. Roberts' Foundations of parasitology (8th ed.). Boston: McGraw-Hill. ISBN 0-07-128458-3.
- ↑ González LM, Montero E, Harrison LJ, Parkhouse RM, Garate T (2000). "Differential diagnosis of Taenia saginata and Taenia solium infection by PCR.". J Clin Microbiol. 38 (2): 737–744. PMC 86191
 . PMID 10655377.
. PMID 10655377. - ↑ Zarlenga DS. (1991). "The differentiation of a newly described Asian taeniid from Taenia saginata using enzymatically amplified non-transcribed ribosomal DNA repeat sequences.". Southeast Asian J Trop Med Public Health. 22 (suppl): 251–255. PMID 1822899.
- ↑ Jimenez JA, Rodriguez S, Moyano LM, Castillo Y, García HH (2010). "Differentiating Taenia eggs found in human stools - Does Ziehl Neelsen staining help?". Tropical Medicine & International Health. 15 (9): 1077–1081. doi:10.1111/j.1365-3156.2010.02579.x.
- ↑ Nkouawa, A; Sako, Y; Li, T; Chen, X; Wandra, T; Swastika, IK; Nakao, M; Yanagida, T; Nakaya, K; Qiu, D; Ito, A (2010). "Evaluation of a loop-mediated isothermal amplification method using fecal specimens for differential detection of Taenia species from humans". Journal of Clinical Microbiology. 48 (9): 3350–2. doi:10.1128/JCM.00697-10. PMC 2937673
 . PMID 20631114.
. PMID 20631114. - ↑ Jeon, Hyeong-Kyu; Eom, Keeseon S. (2009). "Immunoblot Patterns of Taenia asiatica Taeniasis". The Korean Journal of Parasitology. 47 (1): 73–7. doi:10.3347/kjp.2009.47.1.73. PMC 2655338
 . PMID 19290097.
. PMID 19290097. - ↑ "Surveillance, prevention and control of taeniasis/cysticercosis". Retrieved 13 March 2014.
- ↑ http://www.dpd.cdc.gov/DPDx/
- ↑ Lopes WD, Cruz BC, Soares VE, Nunes JL, Teixeira WF, Maciel WG, Buzzulini C, Pereira JC, Felippelli G, Soccol VT, de Oliveira GP, da Costa AJ (2014). "Historic of therapeutic efficacy of albendazol sulphoxide administered in different routes, dosages and treatment schemes, against Taenia saginata cysticercus in cattle experimentally infected". Experimental Parasitology. 137 (1): 14–20. doi:10.1016/j.exppara.2013.11.007. PMID 24309372.
- ↑ Ooi, Hong Kean; Ho, Chau-Mei; Chung, Wen-Cheng (2013). "Historical overview of Taenia asiatica in Taiwan". The Korean Journal of Parasitology. 51 (1): 31–6. doi:10.3347/kjp.2013.51.1.31. PMC 3587746
 . PMID 23467308.
. PMID 23467308. - ↑ Somers, Kenneth D.; Morse, Stephen A. (2010). Lange Microbiology and Infectious Diseases Flash Cards (2nd ed.). New York: Lange Medical Books/ McGraw-Hill. pp. 184–186. ISBN 9780071628792.
- ↑ Ortega, Ynes R. (2006). Foodborne parasites. New York: Springer. pp. 207–210. ISBN 9780387311975.
- ↑ Eom, Keeseon S.; Jeon, Hyeong-Kyu; Rim, Han-Jong (2009). "Geographical distribution of Taenia asiatica and related species". The Korean Journal of Parasitology. 47 (Suppl): S115–24. doi:10.3347/kjp.2009.47.S.S115. PMC 2769216
 . PMID 19885327.
. PMID 19885327. - ↑ Ale, Anita; Victor, Bjorn; Praet, Nicolas; Gabriël, Sarah; Speybroeck, Niko; Dorny, Pierre; Devleesschauwer, Brecht (2014). "Epidemiology and genetic diversity of Taenia asiatica: a systematic review". Parasites & Vectors. 7 (1): 45. doi:10.1186/1756-3305-7-45. PMC 3900737
 . PMID 24450957.
. PMID 24450957. - ↑ Eckert, J. (2005). "Helminths". In Kayser, F.H.; Bienz, K.A.; Eckert, J.; Zinkernagel, R.M. Medical Microbiology. Stuttgart: Thieme. pp. 560–562. ISBN 9781588902450.