Thermal transpiration
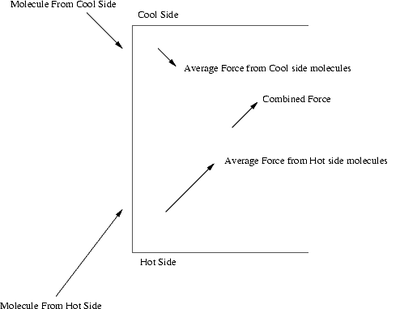
Diagram showing pressure difference induced by a temperature difference.
Thermal transpiration or thermal diffusion refers to the thermal force on a gas due to a temperature difference. Thermal transpiration causes a flow of gas in the absence of any pressure difference, and is able to maintain a certain pressure difference (called thermomolecular pressure difference) in a steady state. The effect is strongest when the mean free path of the gas molecules is comparable to the dimensions of the gas container.
Thermal transpiration appears as an important correction in the readings of vapor pressure thermometers,[1][2] and the effect is historically famous as being an explanation for the rotation of the Crookes radiometer.[3][4]
See also
- Thermophoresis (Soret effect) — diffusion of colloidal particles in a liquid, induced by a temperature gradient.
- Knudsen pump — a gas pump with no moving parts which functions via thermal transpiration.
References
- ↑ Watkins, R. A. (1967). "Thermomolecular Pressure Difference Measurements for Precision Helium−3 and Helium−4 Vapor-Pressure Thermometry". The Journal of Chemical Physics. 46 (3): 1007. Bibcode:1967JChPh..46.1007W. doi:10.1063/1.1840762.
- ↑ Pobell, F. (2007). Matter and Methods at Low Temperatures (3rd ed.). Springer. ISBN 978-3-540-46356-6.
- ↑ "On certain dimensional properties of matter in the gaseous state" Osborne Reynolds, Royal Society Phil. Trans., Part 2, (1879)
- ↑ "On stresses in rarefied gases arising from inequalities of temperature" James Clerk Maxwell, Royal Society Phil. Trans. (1879)
This article is issued from Wikipedia - version of the 5/12/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.