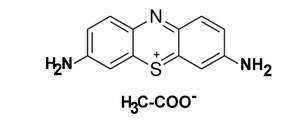
Thionine
 | |
| Identifiers | |
|---|---|
| 135-59-1 | |
| ECHA InfoCard | 100.004.731 |
| PubChem | 65043 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Thionine, also known as thionine acetate or Lauth's violet, is a strongly staining metachromatic dye, featuring a phenothiazine core, that is widely used for biological staining.[1] Thionine can also be used in place of Schiff reagent in quantitative Feulgen staining of DNA. It can also be used to mediate electron transfer in microbial fuel cells.[2] The dye's name is frequently misspelled, with omission of the e. The -ine ending indicates that the compound is an amine.[3][4]
When both the amines are dimethylated, the product tetramethyl thionine is famous as methylene blue, and the intermediates are Azure C (Monomethyl thionine), Azure A (when one of the amines is dimethylated and the other remains primary amine), and Azure B (Trimethyl thionine). When methylene blue is "polychromed" by ripening (Oxidized in solution or metabolized by fungal contamination,[5] as originally noted in the thesis of Dr D L Romanowski in 1890s), it forms thionine and all the Azure intermediates.[6][7]
Notes and references
- ↑ "Stainsfile — Thionin".
- ↑ Eugenii Katz; Andrew N. Shipway; Itamar Willner (2003). "21". In Wolf Vielstich. Handbook of Fuel Cells: Fundamentals, Technology, Applications, 4-Volume Set (PDF). Wiley. p. 5. ISBN 978-0-471-49926-8.
- ↑ Kiernan JA (2001). "Classification and naming of dyes, stains and fluorochromes". Biotech Histochem. 76 (5-6): 261–78. doi:10.1080/bih.76.5-6.261.278. PMID 11871748.
- ↑ Webster's Third New International Dictionary. G & C Merriam Co. 1976, p.2377.
- ↑ Dako Education Guide - Special Stains and H & E ” second edition Chapter 19: On Chemical Reactions and Staining Mechanisms by John A. Kiernan, Subsection What is Giemsa’s stain and how does it color blood cells, bacteria and chromosomes? p172
- ↑ J Exp Med. 1907 Nov 1;9(6):645-70. ON THE CHEMISTRY AND STAINING PROPERTIES OF CERTAIN DERIVATIVES OF THE METHYLENE BLUE GROUP WHEN COMBINED WITH EOSIN. Wilson TM.
- ↑ Marshall,PN (1978) Romanowsky-type stains in haematology. Histochemical Journal 10: 1-29.