Thiourea dioxide
 | |||
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
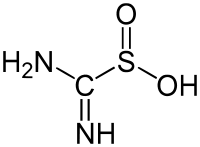
amino(imino)methanesulfinic acid | |||
| Other names
Thiourea dioxide, DegaFAS, Reducing Agent F, Depilor, Formamidine Sulfinic Acid | |||
| Identifiers | |||
| 1758-73-2 | |||
| 3D model (Jmol) | Interactive image | ||
| ECHA InfoCard | 100.015.598 | ||
| PubChem | 61274 | ||
| |||
| Properties | |||
| CH4N2O2S | |||
| Molar mass | 108.12 g·mol−1 | ||
| Appearance | White powder | ||
| Melting point | 126 °C (259 °F; 399 K) | ||
| 3.0 g/100 mL | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Thiourea dioxide or thiox is an organosulfur compound that is used in the textile industry.[1] It functions as a reducing agent.[2] Thiourea dioxide is not a dioxide, but instead is a derivative of a sulfinic acid (RS(O)(OH), hence the alternative name formamidine sulfinic acid.[3]
Synthesis
Thiourea dioxide is prepared by the oxidation of thiourea with hydrogen peroxide.[4]
- (NH2)2CS + 2H2O2 → (NH)(NH2)CSO2H + 2H2O
The mechanism of the oxidation has been examined.[5] An aqueous solution of thiourea dioxide has a pH about 6.5 at which thiourea dioxide is hydrolyzed to urea and sulfoxylic acid. It has been found that at pH values of less than 2, thiourea and hydrogen peroxide react to form a disulfide species. It is therefore convenient to keep the pH between 3 and 5 and the temperature below 10 °C.[6] It can also be prepared by oxidation of thiourea with chlorine dioxide.[7] The quality of the product can be assessed by titration with indigo.[4]
Uses
Thiourea dioxide is used in reductive bleaching in textiles.[8] Thiourea dioxide has also been used for the reduction of aromatic nitroaldehydes and nitroketones to nitroalcohols.[9]
Structure
The structure of thiourea dioxide is related to that for thiourea. The N2CS core is approximately planar, but the sulfur is pyramidal. Selected bond lengths: S-C = 1.85, C-N = 1.31, and S-O = 1.49 Å.[3]
References
- ↑ Klaus Fischer et al. "Textile Auxiliaries" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a26_227
- ↑ Milne, G. W. A. Dictionary, in Gardner's Commercially Important Chemicals: Synonyms, Trade Names, and Properties, John Wiley & Sons, Inc. 2005, Hoboken, NJ, USA. doi:10.1002/0471736627.ch1.
- 1 2 Sullivan, R. A. L.; Hargreaves, A. (1962). "The Crystal and Molecular Structure of Thiourea Dioxide". Acta Crystallographica. 15 (7): 675–682. doi:10.1107/S0365110X62001851.
- 1 2 D. Schubart "Sulfinic Acids and Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry, 2012, Wiley-VCH, Weinheim. doi:10.1002/14356007.a25_461
- ↑ Hoffmann, Michael; Edwards, John O. (1977). "Kinetics and Mechanism of the Oxidation of Thiourea and N,N'-dialkylthioureas by Hydrogen Peroxide". Inorganic Chemistry. 16: 3333–3338. doi:10.1021/ic50178a069.
- ↑ US patent 2783272, James H. Young, "PRODUCTION OF FORMAMIDINE SULFINIC ACID", issued 1957-2-26
- ↑ Rábai, G., Wang, R. T. and Kustin, "Kinetics and mechanism of the oxidation of thiourea by chlorine dioxide" International Journal of Chemical Kinetics, 1993, volume 25: 53–62. doi:10.1002/kin.550250106.
- ↑ Hebeish, A., El-Rafie, M. H., Waly, A. and Moursi, A. Z. (1978). "Graft copolymerization of vinyl monomers onto modified cotton. IX. Hydrogen peroxide–thiourea dioxide redox system induced grafting of 2-methyl-5-vinylpyridine onto oxidized celluloses". Journal of Applied Polymer Science. 22 (7): 1853–1866. doi:10.1002/app.1978.070220709.
- ↑ Sambher, S., Baskar, C., & Dhillon, R. S. (2009). "Chemoselective reduction of carbonyl groups of aromatic nitro carbonyl compounds to the corresponding nitroalcohols using thiourea dioxide". Arkivoc. 10: 141–145. ISSN 1551-7012.

