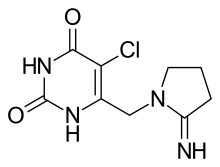
Tipiracil
 | |
| Pharmacokinetic data | |
|---|---|
| Bioavailability | ≥27% |
| Protein binding | <8% |
| Biological half-life | 2.1–2.4 hrs |
| Excretion | Faeces (50%), urine (27%) |
| Identifiers | |
| |
| CAS Number |
183204-74-2 183204-72-0 (HCl) |
| PubChem (CID) | 6323266 |
| DrugBank | DB09343 |
| ChemSpider | 13243748 |
| KEGG | D10467 |
| ChEBI |
CHEBI:90879 |
| ChEMBL | CHEMBL235668 |
| Chemical and physical data | |
| Formula | C9H11ClN4O2 |
| Molar mass | 242.67 g/mol |
| 3D model (Jmol) | Interactive image |
| Solubility in water | 5 mg/mL (20 °C) |
| |
| |
Tipiracil is a drug used in the treatment of cancer. It is approved for use in form of the combination drug trifluridine/tipiracil for the treatment of unresectable advanced or recurrent colorectal cancer.[1]
Tipiracil helps maintain the blood concentration of trifluridine by inhibiting the enzyme thymidine phosphorylase which metabolizes trifluridine.[1][2]
Adverse effects
Adverse effects were not assessed independently of trifluridine, but only in the combination drug.
Interactions
Only in vitro interaction studies are available. In these, tipiracil was transported by the solute carrier proteins SLC22A2 and SLC47A1. Drugs that interact with these transporters could influence blood plasma concentrations of tipiracil.[3]
Pharmacology
Mechanism of action
Tipiracil is a thymidine phosphorylase (TPase) inhibitor and inhibits degradation of trifluridine by inhibiting TPase, thus increasing systemic exposure to trifluridine when tipiracil is given together with trifluridine.[3]
Pharmacokinetics
At least 27% of tipiracil is absorbed from the gut. In cancer patients, highest blood plasma concentrations are reached after three hours. The substance has no tendency to accumulate in the body. The in vitro protein binding in human plasma is below 8%. Tipiracil is not metabolized by cytochrome P450 (CYP) enzymes. To a small extent, it is hydrolyzed to 6-hydroxymethyluracil, but the main fraction is excreted in unchanged form in the faeces (50%) and urine (27%). Elimination half-life is 2.1 hours on the first day and then slightly increases to 2.4 hours on the twelfth day.[3][4]
Tipiracil causes Cmax (highest blood plasma concentrations) of trifluridine to increase 22-fold, and its area under the curve 37-fold.[3]
Chemistry
Tipiracil is used in form of the hydrochloride,[3] which is a white crystalline powder. Solubility in water is 5 mg/mL;[5] it is also soluble in 0.01 M hydrochloric acid and 0.01 M sodium hydroxide; slightly soluble in methanol; very slightly soluble in ethanol; and practically insoluble in acetonitrile, isopropyl alcohol, acetone, diisopropyl ether, and diethyl ether.[6]
References
- 1 2 "Taiho's Lonsurf(R) (trifluridine and tipiracil hydrochloride) Tablets Approved In Japan for Treatment of Advanced Metastatic Colorectal Cancer". March 24, 2014.
- ↑ Tanaka, N; Sakamoto, K; Okabe, H; Fujioka, A; Yamamura, K; Nakagawa, F; Nagase, H; Yokogawa, T; Oguchi, K; Ishida, K; Osada, A; Kazuno, H; Yamada, Y; Matsuo, K (2014). "Repeated oral dosing of TAS-102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models". Oncology Reports. 32 (6): 2319–26. doi:10.3892/or.2014.3487. PMC 4240496
 . PMID 25230742.
. PMID 25230742. - 1 2 3 4 5 Haberfeld, H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
- ↑ "Lonsurf: EPAR – Product Information" (PDF). European Medicines Agency. 12 May 2016.
- ↑ "Tipiracil hydrochloride". Sigma Aldrich. Retrieved 28 September 2016.
- ↑ FDA Professional Drug Information on Lonsurf.