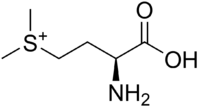
S-Methylmethionine
 | |
| Names | |
|---|---|
| IUPAC name
(3-Amino-3-carboxy-propyl)-dimethyl-sulfonium | |
| Other names
S-Methyl-L-methionine Vitamin U | |
| Identifiers | |
| 4727-40-6 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:17728 |
| ChemSpider | 128519 |
| KEGG | C03172 |
| PubChem | 145692 |
| |
| |
| Properties | |
| C6H14NO2S+ | |
| Molar mass | 164.243 g/mol |
| Melting point | 139 °C (282 °F; 412 K)[1] (bromide salt, decomposes) 134 °C (273 °F)[1] (chloride salt, decomposes) |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
2760 mg/kg (iv, mice, chloride salt)[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
S-Methylmethionine (SMM) is a derivative of methionine with the chemical formula (CH3)2S+CH2CH2CH(NH3+)CO2−. This cation is an intermediate in many biosynthetic pathways owing to the sulfonium functional group. The natural derivative S-methylmethionine is biosynthesized from L-methionine which is first converted to S-adenosylmethionine. The subsequent conversion, involving replacement of the adenosyl group by a methyl group is catalyzed by the enzyme methionine S-methyltransferase. S-methylmethionine is particularly abundant in plants, being more abundant than methionine.[2]
S-Methylmethionine is sometimes referred to as vitamin U,[3] but it is not considered a true vitamin. The term was coined in 1950 by Garnett Cheney for uncharacterized anti-ulcerogenic[4] factors in raw cabbage juice that may help speed healing of peptic ulcers.
S-Methylmethionine is claimed to have protective effects in the gastrointestinal mucosa and in the liver.[5]
Biosynthesis and biochemical function
S-Methylmethionine arises via the methylation of methionine by S-adenosyl methionine (SAM). The coproduct is S-adenosyl homocysteine.[2]
The biological roles of S-methylmethionine are not well understood. Speculated roles include methionine storage, use as a methyl donor, regulation of SAM.[2] A few plants use S-methylmethionine as a precursor to the osmolyte dimethylsulfoniopropionate (DMSP). Intermediates include dimethylsulfoniumpropylamine and dimethylsulfoniumpropionaldehyde.[6]
Beer flavor precursor in barley malt
S-Methylmethionine is found in barley and during the malting process, particularly the curing stage in kilning, heat causes it to break down to form dimethyl sulfide (DMS).[7]
References
- 1 2 3 4 Merck Index, 12th ed., 10165 ISBN 0-911910-12-3
- 1 2 3 Bourgis F (1999). "S-methylmethionine plays a major role in phloem sulfur transport and is synthesized by a novel type of methyltransferase". The Plant Cell Online. 11 (8): 1485–1498. doi:10.1105/tpc.11.8.1485.
- ↑ National Center for Biomedical Ontology. "Methylmethionine Sulfonium Chloride".
- ↑ Cheney G (September 1950). "Anti-peptic ulcer dietary factor (vitamin "U") in the treatment of peptic ulcer". J Am Diet Assoc. 26 (9): 668–72. PMID 15436263.
- ↑ Patel AD, Prajapati NK (2012). "Review on Biochemical Importance of Vitamin-U" (PDF). Journal of Chemical and Pharmaceutical Research. 4 (1): 209–215.
- ↑ McNeil SD (1999). "Betaines and related osmoprotectants. Targets for metabolic engineering of stress resistance". Plant Physiology. 120 (4): 945–949. doi:10.1104/pp.120.4.945.
- ↑ Hornsey IS (1999). Brewing. Royal Society of Chemistry. p. 47. ISBN 9780854045686.
External links
- Vitamin U at the US National Library of Medicine Medical Subject Headings (MeSH)