Westphalen–Lettré rearrangement
The Westphalen–Lettré rearrangement is a classic organic reaction in organic chemistry describing a rearrangement reaction of cholestane-3β,5α,6β-triol diacetate with acetic anhydride and sulfuric acid. In this reaction one equivalent of water is lost, a double bond is formed at C10–C11 and importantly the methyl group at the C10 position migrates to the C5 position.[1][2][3]
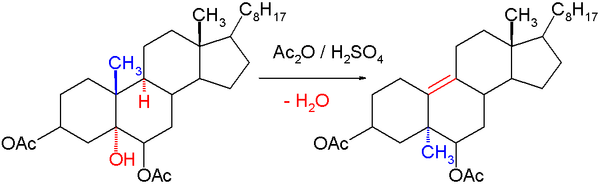
The reaction is first-order in steroid in the presence of an excess of sulfuric acid[4] and the first reaction step in the reaction mechanism is likely the formation of an sulfate ester followed by that of a carbocation at C5 after which the actual rearrangement takes place.
References
- ↑ Theodor Westphalen, Ber., 48, 1064 (1915) doi:10.1002/cber.191504801149
- ↑ H. Lettré and I. Muller, Ber., 70, 1947 (1937) doi:10.1002/cber.19370700918
- ↑ Rearranged Steroid Systems. I. Studies in the Pregnane Series O. R. RODIG, P. BROWN, and P. ZAFFARONI J. Org. Chem. 1961, 26(7), 2431–2435. (doi: 10.1021/jo01351a066)
- ↑ Acid catalysed reactions of 5α-hydroxy-steroids—III : The westphalen rearrangementTetrahedron, Volume 21, Issue 6, 1965, Pages 1567–1580 J. W. Blunt, A. Fischer, M. P. Hartshorn, F. W. Jones, Kirk D. N. and S. W. Yoong (doi:10.1016/S0040-4020(01)98321-8)
This article is issued from Wikipedia - version of the 4/20/2014. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.