Wien's displacement law
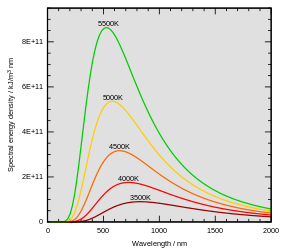
Wien's displacement law states that the black body radiation curve for different temperatures peaks at a wavelength inversely proportional to the temperature. The shift of that peak is a direct consequence of the Planck radiation law which describes the spectral brightness of black body radiation as a function of wavelength at any given temperature. However it had been discovered by Wilhelm Wien several years before Max Planck developed that more general equation, and describes the entire shift of the spectrum of black body radiation toward shorter wavelengths as temperature increases.
Formally, Wien's displacement law states that the spectral radiance of black body radiation per unit wavelength, peaks at the wavelength λmax given by:
where T is the absolute temperature in kelvin. b is a constant of proportionality called Wien's displacement constant, equal to 2.8977729(17)×10−3 m K[1], or more conveniently to obtain wavelength in micrometers, b ≈ 2900 μm·K. If one is considering the peak of black body emission per unit frequency or per proportional bandwidth, one must use a different proportionality constant. However the form of the law remains the same: the peak wavelength is inversely proportional to temperature (or the peak frequency is directly proportional to temperature).
Wien's displacement law may be referred to as "Wien's law", a term which is also used for the Wien approximation.
Examples
Wien's displacement law is relevant to some everyday experiences:
- A piece of metal heated by a torch first becomes "red hot" as the very longest visible wavelengths appear red, then becomes more orange-red as the temperature is increased, and at very high temperatures would be described as "white hot" as shorter and shorter wavelengths come to predominate the black body emission spectrum. Before it had even reached the red hot temperature, the thermal emission was mainly at longer infrared wavelengths which are not visible; nevertheless that radiation could be felt as it warms one's nearby skin.
- One easily observes changes in the color of an incandescent light bulb (which produces light through black body radiation) as the temperature of its filament is varied by a light dimmer. As the light is dimmed and the filament temperature decreases, the distribution of color shifts toward longer wavelengths and the light appears redder, as well as dimmer.
- It is easy to calculate that a wood fire at 1500 K puts out peak radiation at about 2000 nm. 98% of its radiation is beyond 1000 nm and only a tiny proportion at visible wavelengths. Consequently, a campfire can keep one warm but is a poor source of visible light.
- The effective temperature of the Sun is 5778 K. Using Wien's law, one finds a peak emission per nanometer (of wavelength) at a wavelength of about 500 nm in the green portion of the spectrum near the peak sensitivity of the human eye.[2][3] On the other hand, in terms of power per unit optical frequency, the Sun's peak emission is at 343 THz or a wavelength of 883 nm in the near infrared. In terms of power per percentage bandwidth, the peak is at about 635 nm, a red wavelength. Regardless of how one wants to plot the spectrum, about half of the sun's radiation is at wavelengths shorter than 710 nm, about the limit of the human vision. Of that, about 12% is at wavelengths shorter than 400 nm, ultraviolet wavelengths which cannot be seen. It can be appreciated that a rather large amount of the Sun's radiation falls in the fairly small visible spectrum.

- The preponderance of emission in the visible range, however, is not the case in most stars. The hot supergiant Rigel emits 60% of its light in the ultraviolet, while the cool supergiant Betelgeuse emits 85% of its light at infrared wavelengths. With both stars prominent in the constellation of Orion, one can easily appreciate the color difference between the blue-white Rigel (T = 12100 K) and the red Betelgeuse (T ≈ 3300 K). While few stars are as hot as Rigel, stars cooler than the sun or even as cool as Betelgeuse are very commonplace.
- Mammals with a skin temperature of about 300 K emit peak radiation at around 10 μm in the far infrared. This is therefore the range of infrared wavelengths that pit viper snakes and passive IR cameras must sense.
- When comparing the apparent color of lighting sources (including fluorescent lights, LED lighting, computer monitors, and photoflash), it is customary to cite the color temperature. Although the spectra of such lights are not accurately described by the black body radiation curve, a color temperature is quoted for which black body radiation would most closely match the subjective color of that source. For instance, the blue-white fluorescent light sometimes used in an office may have a color temperature of 6500 K, whereas the reddish tint of a dimmed incandescent light may have a color temperature (and an actual filament temperature) of 2000 K. Note that the informal description of the former (bluish) color as "cool" and the latter (reddish) as "warm" is exactly opposite the actual temperature change involved in black body radiation.
Discovery
The law is named for Wilhelm Wien, who derived it in 1893 based on a thermodynamic argument.[4] Wien considered adiabatic expansion of a cavity containing waves of light in thermal equilibrium. He showed that under slow expansion or contraction, the energy of light reflecting off the walls changes in exactly the same way as the frequency. A general principle of thermodynamics is that a thermal equilibrium state, when expanded very slowly stays in thermal equilibrium. The adiabatic principle allowed Wien to conclude that for each mode, the adiabatic invariant energy/frequency is only a function of the other adiabatic invariant, the frequency/temperature. A modern variant of Wien's derivation can be found in the textbook by Wannier.[5]
The consequence is that the shape of the black body radiation function (which was not yet understood) would shift proportionally in frequency (or inversely proportionally in wavelength) with temperature. When Max Planck later formulated the correct black body radiation function it did not include Wien's constant b explicitly. Rather, Planck's constant h was created and introduced into his new formula. From Planck's constant h and the Boltzmann constant k, Wien's constant b can be obtained.
Frequency-dependent formulation
For spectral flux considered per unit frequency (in hertz), Wien's displacement law describes a peak emission at the optical frequency given by:
where α ≈ 2.821439... is a constant resulting from the numerical solution of the maximization equation, k is the Boltzmann constant, h is the Planck constant, and T is the temperature (in kelvin). This frequency does not correspond to the wavelength from the earlier formula which considered the peak emission per unit wavelength.
Derivation from Planck's Law
Planck's law for the spectrum of black body radiation predicts the Wien displacement law and may be used to numerically evaluate the constant relating temperature and peak wavelength (or frequency). According to one form of that law, the black body spectral radiance (power per emitting area per solid angle per unit wavelength) is given by:
Differentiating u(λ,T) with respect to λ and setting the derivative equal to zero gives
which can be simplified to give
By defining:
the equation becomes one in that single variable:
The numerical solution to this equation is[note 1] x = 4.965114231.
Solving for the wavelength λ in units of nanometers, and using kelvin for the temperature yields:
The form of Wien's displacement law in terms of maximum radiance per unit frequency is derived using similar methods, but starting with the form of Planck's law expressed in those terms rather than wavelength. The effective result is to substitute 3 for 5 in the equation for the peak wavelength. This is solved with x = 2.821439372.
Using the value 4 in this equation solves for the wavelength of the peak in the spectral radiance expressed in radiance per proportional bandwidth, perhaps a fairer way of presenting "wavelength of peak emission." That is solved as x = 3.920690395. The important point of Wiens law, however, is that any such wavelength marker, including the median wavelength (or the wavelength below which a specified percentage of the emission occurs) is proportional to the reciprocal of temperature.
See also
Notes
- ↑ The equation cannot be solved in terms of elementary functions. It can be solved in terms of Lambert's product log function as , but the exact solution is not important in this derivation.
References
- ↑ "CODATA Value: Wien wavelength displacement law constant". The NIST Reference on Constants, Units, and Uncertainty. US National Institute of Standards and Technology. June 2015. Retrieved 2015-09-25.
- ↑ Walker, J. Fundamentals of Physics, 8th ed., John Wiley and Sons, 2008, p. 891. ISBN 9780471758013.
- ↑ Feynman, R; Leighton, R; Sands,M. The Feynman Lectures on Physics, vol. 1, pp. 35-2 – 35-3. ISBN 0201510030.
- ↑ Mehra, J.; Rechenberg, H. (1982). The Historical Development of Quantum Theory. New York: Springer-Verlag. Chapter 1. ISBN 978-0-387-90642-3.
- ↑ Wannier, G. H. (1987) [1966]. Statistical Physics. Dover Publications. Chapter 10.2. ISBN 978-0-486-65401-0. OCLC 15520414.
Further reading
- Soffer, B. H.; Lynch, D. K. (1999). "Some paradoxes, errors, and resolutions concerning the spectral optimization of human vision". American Journal of Physics. 67 (11): 946–953. Bibcode:1999AmJPh..67..946S. doi:10.1119/1.19170.
- Heald, M. A. (2003). "Where is the 'Wien peak'?". American Journal of Physics. 71 (12): 1322–1323. Bibcode:2003AmJPh..71.1322H. doi:10.1119/1.1604387.