Wohl–Aue reaction
The Wohl–Aue reaction is an organic reaction between an aromatic nitro compound and an aniline to form a phenazine in presence of an alkali base.[1][2] An example is the reaction between nitrobenzene and aniline:
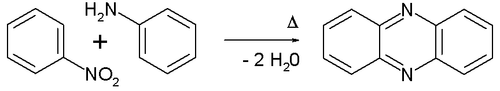
The reaction is named after Alfred Wohl and W. Aue.
References
- ↑ Alfred Wohl and W. Aue (1901). "Über die Einwirkung von Nitrobenzol auf Anilin bei Gegenwart von Alkali". Chemische Berichte. 34 (2): 2442–2450. doi:10.1002/cber.190103402183.
- ↑ Irwin J. Pachter and Milton C. Kloetzel (1951). "The Wohl-Aue Reaction. I. Structure of Benzo [a] phenazine Oxides and Syntheses of 1,6-Dimethoxyphenazine and 1,6-Dichlorophenazine". J. Am. Chem. Soc. 73 (10): 4958–4961. doi:10.1021/ja01154a144.
This article is issued from Wikipedia - version of the 4/20/2013. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.