5,10-Methylenetetrahydrofolate
 | |
| Names | |
|---|---|
| IUPAC name
N-[4-(3-amino-1-oxo-1,4,5,6,6a,7-hexahydroimidazo[1,5-f]pteridin-8(9H)-yl)benzoyl]-L-glutamic acid | |
| Other names
5,10-CH2-THF, MTHF | |
| Identifiers | |
| 3432-99-3 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:20502 |
| ChEMBL | ChEMBL117348 |
| ChemSpider | 97272 |
| MeSH | 5,10-methylenetetrahydrofolate |
| PubChem | 108194 |
| |
| |
| Properties | |
| C20H23N7O6 | |
| Molar mass | 457.44 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
5,10-Methylenetetrahydrofolate (N5,N10-Methylenetetrahydrofolate; 5,10-CH2-THF) is the substrate used by the enzyme methylenetetrahydrofolate reductase (MTHFR)[1][2] to generate 5-methyltetrahydrofolate (5-MTHF, or levomefolic acid).
5,10-CH2-THF can also be used as a coenzyme in the biosynthesis of thymidine. To be specific, it is the C1-donor in the reactions catalyzed by thymidylate synthase and thymidylate synthase (FAD). It also acts as a cofactor in the synthesis of serine from glycine via the enzyme serine hydroxymethyltransferase.
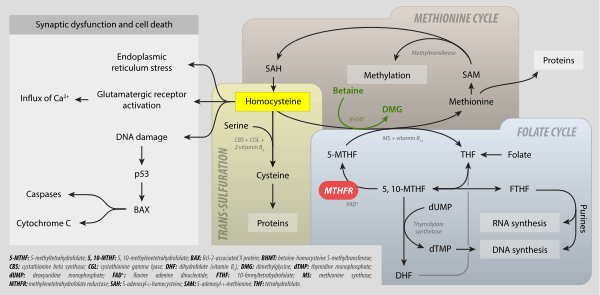
MTHFR metabolism: folate cycle, methionine cycle, trans-sulfuration and hyperhomocysteinemia. 5-MTHF: 5-methyltetrahydrofolate; 5,10-methyltetrahydrofolate; BAX: Bcl-2-associated X protein; BHMT: betaine-homocysteine S-methyltransferase; CBS: cystathionine beta synthase; CGL: cystathionine gamma-lyase; DHF: dihydrofolate (vitamin B9); DMG: dimethylglycine; dTMP: thymidine monophosphate; dUMP: deoxyuridine monophosphate; FAD+ flavine adenine dicucleotide; FTHF: 10-formyltetrahydrofolate; MS: methionine synthase; MTHFR: mehtylenetetrahydrofolate reductase; SAH: S-adenosyl-L-homocysteine; SAME: S-adenosyl-L-methionine; THF: tetrahydrofolate.
See also
References
- ↑ "Entrez Gene: MTHFR methylenetetrahydrofolate reductase (NAD(P)H)".
- ↑ Födinger M, Hörl WH, Sunder-Plassmann G (2000). "Molecular biology of 5,10-methylenetetrahydrofolate reductase.". J Nephrol. 13 (1): 20–33. PMID 10720211.
This article is issued from Wikipedia - version of the 7/20/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.