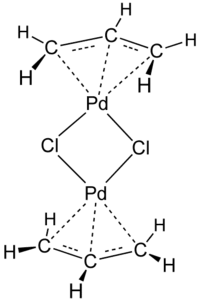
Allylpalladium chloride dimer
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Allylpalladium(II) chloride dimer | |
| Other names
Allylpalladium chloride dimer bis(allyl)di-μ-chloro-dipalladium(II) APC | |
| Identifiers | |
| 12012-95-2 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 21171401 |
| ECHA InfoCard | 100.031.423 |
| PubChem | 61538 |
| |
| |
| Properties | |
| C6H10Cl2Pd2 | |
| Molar mass | 365.85 g/mol |
| Appearance | Pale yellow, crystalline solid |
| Density | Solid |
| Melting point | decomp at 155-156 °C |
| Insoluble | |
| Solubility in other solvents | Chloroform benzene acetone methanol |
| Hazards | |
| Safety data sheet | http://www.colonialmetals.com/pdf/5048.pdf |
| R-phrases | 36/37/38 |
| S-phrases | 26-36 |
| Related compounds | |
| Related compounds |
(η3-allyl)(η5 – cyclopentadienyl)palladium(II) di-μ-chlorobis(crotyl)dipalladium |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Allylpalladium(II) chloride dimer is a chemical compound with the formula (η3- C3H5)2Pd2Cl2. This yellow air-stable compound is an important catalyst used in organic synthesis.[1] It is one of the most widely used transition metal allyl complexes.
Synthesis and reactions
The compound is prepared by purging carbon monoxide through a methanolic aqueous solution of palladium(II) chloride, sodium chloride, and allyl chloride.[1]
- 2Na2PdCl4 + 2 CH2=CHCH2Cl + 2 CO + 2 H2O → (C3H5)2Pd2Cl2 + 4 NaCl + 2 CO2 + 4 HCl
APC reacts with sources of cyclopentadienyl anion to give the corresponding 18e complex cyclopentadienyl allyl palladium:
- (C3H5)2Pd2Cl2 + 2 NaC5H5 → 2 Pd(η3-C3H5)(η5-C5H5) + 2 NaCl
References
- 1 2 Tatsuno, Y.; Yoshida, T.; Otsuka, S. "(η3-allyl)palladium(II) Complexes" Inorganic Syntheses, 1990, volume 28, pages 342-345. ISBN 0-471-52619-3
This article is issued from Wikipedia - version of the 10/9/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.