Transition metal allyl complex
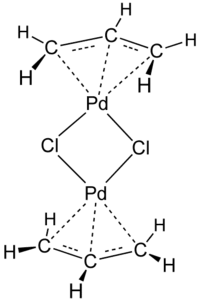
Transition metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH-CH2-, which is usually described as two equivalent resonance structures.
Examples and their syntheses
The allyl ligand is commonly in organometallic chemistry. Most commonly, allyl ligands bind to metals via all three carbon centers, the η3-binding mode. An example of a homoleptic allyl complex is Ir(η3-allyl)3 Mixed ligand allyl complexes are more common. Examples include (η3-allyl)Mn(CO)4 and CpPd(allyl).
Allyl complexes are usually generated by oxidative addition of allylic halides to low-valent metal complexes. This route is used to prepare (allyl)2Ni2Cl2:[1]
- 2 Ni(CO)4 + 2 ClCH2CH=CH2 → Ni2(μ-Cl)2(η3-C3H5)2 + 8 CO
Sigma-allyl and benzyl complexes
Some complexes with η1-allyl ligands are also known, e.g., CpFe(CO)2(η1-allyl) where only the methylene group is attached to the Fe centre. Such compounds often convert to the η3-allyl derivatives by dissociation of a ligand:
- CpFe(CO)2(η1-allyl) → CpFe(CO) (η3-allyl) + CO
Benzyl and allyl compounds often exhibit similar chemical properties. Similarly, benzyl is a common ligand. Unlike allyl, benzyl favors the η1 bonding mode.
Applications
In terms of applications, a popular allyl complex is allyl palladium chloride.[2] Allyl ligands are susceptible to nucleophilic addition, which can be useful in organic synthesis.[3]
References
- ↑ Martin F. Semmelhack and Paul M. Helquist (1988). "Reaction of Aryl Halides with π-Allylnickel Halides: Methallylbenzene". Org. Synth.; Coll. Vol., 6, p. 722
- ↑ Tatsuno, Y.; Yoshida, T.; Otsuka, S. "(η3-allyl)palladium(II) Complexes" Inorganic Syntheses, 1990, volume 28, pages 342-345. ISBN 0-471-52619-3
- ↑ Hartwig, J. F. Organotransition Metal Chemistry, from Bonding to Catalysis; University Science Books: New York, 2010. ISBN 189138953X