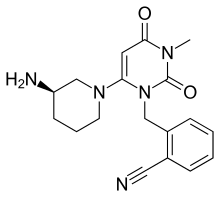
Alogliptin
 | |
| Clinical data | |
|---|---|
| Trade names |
Nesina, Vipidia Kazano, Vipidomet (with metformin) Oseni, Incresync (with pioglitazone) |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | A10BH04 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% |
| Protein binding | 20% |
| Metabolism | Limited, hepatic (CYP2D6- and 3A4-mediated) |
| Biological half-life | 12–21 hours |
| Excretion | Renal (major) and fecal (minor) |
| Identifiers | |
| |
| Synonyms | SYR-322 |
| CAS Number |
850649-62-6 |
| PubChem (CID) | 11450633 |
| IUPHAR/BPS | 6319 |
| ChemSpider |
9625485 |
| UNII |
JHC049LO86 |
| KEGG |
D06553 |
| ChEBI |
CHEBI:72323 |
| ChEMBL |
CHEMBL376359 |
| Chemical and physical data | |
| Formula | C18H21N5O2 |
| Molar mass | 339.39 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Alogliptin (trade name Nesina in the US[1] and Vipidia in Europe[2]) is an orally administered anti-diabetic drug in the DPP-4 inhibitor class,[3] developed by Syrrx, a company which was acquired by Takeda Pharmaceutical Company in 2005.[4] Like other medications for the treatment of Type 2 diabetes, alogliptin does not decrease the risk of heart attack and stroke. Like other members of the gliptin class, it causes little or no weight gain, exhibits relatively little risk of causing hypoglycemia, and exhibits relatively modest glucose-lowering activity. Alogliptin and other gliptins are commonly used in combination with metformin in patients whose diabetes cannot adequately be controlled with metformin alone.[5] In April 2016, the U.S. FDA added a warning about increased risk of heart failure.[6]
Medical uses
Alogliptin is a dipeptidyl peptidase-4 inhibitor that decreases blood sugar similar to the other.[7]
Side effects
Adverse events include mild hypoglycemia based on clinical studies.[8][9][10] It may cause severe joint pain.[11]
Alogliptin is not associated with increased weight, increased risk of cardiovasular events.[12][13] In April 2016, the U.S. FDA added a warning about increased risk of heart failure.[6]
Market access
In December 2007, Takeda submitted a New Drug Application (NDA) for alogliptin to the United States Food and Drug Administration (USFDA),[14] after positive results from Phase III clinical trials.[1] In September 2008, the company also filed for approval in Japan,[15] winning approval in April 2010.[14] The company also filed a Marketing Authorization Application (MAA) elsewhere outside the United States, which was withdrawn in June 2009 needing more data.[15] The first USFDA NDA failed to gain approval and was followed by a pair of NDAs (one for alogliptin and a second for a combination of alogliptin and pioglitazone) in July 2011.[14] In 2012, Takeda received a negative response from the USFDA on both of these NDAs, citing a need for additional data.[14]
In 2013 the FDA approved the drug in three formulations: As a stand-alone with the brand-name Nesina. Combined with metformin using the name Kazano, and when combined with pioglitazone as Oseni.
See also
References
- 1 2 "Takeda Submits New Drug Application for Alogliptin (SYR-322) in the U.S." (Press release). Takeda Pharmaceutical Company. January 4, 2008. Retrieved January 9, 2008.
- ↑ Vipidia: EPAR summary for the public (European Medicines Agency)
- ↑ Feng, Jun; Zhang, Zhiyuan; Wallace, Michael B.; Stafford, Jeffrey A.; Kaldor, Stephen W.; Kassell, Daniel B.; Navre, Marc; Shi, Lihong; Skene, Robert J.; Asakawa, Tomoko; Takeuchi, Koji; Xu, Rongda; Webb, David R.; Gwaltney II, Stephen L. (2007). "Discovery of alogliptin: a potent, selective, bioavailable, and efficacious inhibitor of dipeptidyl peptidase IV". J. Med. Chem. 50 (10): 2297–2300. doi:10.1021/jm070104l. PMID 17441705.
- ↑
- ↑ "www.aace.com" (PDF).
- 1 2 "Safety Alerts for Human Medical Products - Diabetes Medications Containing Saxagliptin and Alogliptin: Drug Safety Communication - Risk of Heart Failure". www.fda.gov. Retrieved 7 April 2016.
- ↑ Saisho, Y (2015). "Alogliptin benzoate for management of type 2 diabetes.". Vascular health and risk management. 11: 229–43. doi:10.2147/VHRM.S68564. PMID 25914541.
- ↑ Seino, Yutaka; Fujita, Tetsuya; Hiroi, Shinzo; Hirayama, Masashi; Kaku, Kohei (September 2011), "Efficacy and safety of alogliptin in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, dose-ranging comparison with placebo, followed by a long-term extension study (abstract only)", Current Medical Research and Opinion, 27 (9): 1781–1792, doi:10.1185/03007995.2011.599371, PMID 21806314, retrieved April 26, 2012
- ↑ Kutoh, Eiji; Ukai, Yasuhiro (2012), "Alogliptin as an initial therapy in patients with newly diagnosed, drug naïve type 2 diabetes: a randomized, control trial (abstract only)", Endocrine (published January 17, 2012), 41: 435–41, doi:10.1007/s12020-012-9596-0, PMID 22249941, retrieved April 26, 2012
- ↑ Bosi, Emanuele; Ellis, G.C.; Wilson, C.A.; Fleck, P.R. (October 2011), "Alogliptin as a third oral antidiabetic drug in patients with type 2 diabetes and inadequate glycaemic control on metformin and pioglitazone: a 52-week, randomized, double-blind, active-controlled, parallel-group study", Diabetes, Obesity and Metabolism (published October 27, 2011), 13 (12): 1088–1096, doi:10.1111/j.1463-1326.2011.01463.x, retrieved April 26, 2012
- ↑ "DPP-4 Inhibitors for Type 2 Diabetes: Drug Safety Communication - May Cause Severe Joint Pain". FDA. 2015-08-28. Retrieved 1 September 2015.
- ↑ White WB, Cannon CP, Heller SR, et al. (October 2013). "Alogliptin after acute coronary syndrome in patients with type 2 diabetes". N. Engl. J. Med. 369 (14): 1327–35. doi:10.1056/NEJMoa1305889. PMID 23992602.
- ↑ White WB, Zannad F (January 2014). "Saxagliptin, alogliptin, and cardiovascular outcomes". N. Engl. J. Med. 370 (5): 484. doi:10.1056/NEJMc1313880. PMID 24482824.
- 1 2 3 4 Grogan, Kevin (April 26, 2012), "FDA wants yet more data on Takeda diabetes drug alogliptin", PharmaTimes, PharmaTimes, PharmaTimes online, retrieved April 26, 2012
- 1 2 "GEN News Highlights: Takeda Pulls MAA for Type 2 Diabetes Therapy". Genetic Engineering & Biotechnology News. June 4, 2009.