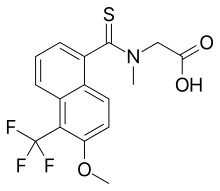
Tolrestat
 | |
 | |
| Clinical data | |
|---|---|
| ATC code | A10XA01 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
82964-04-3 |
| PubChem (CID) | 53359 |
| IUPHAR/BPS | 7404 |
| ChemSpider |
48194 |
| UNII |
0T93LG5NMK |
| KEGG |
D02323 |
| ChEMBL |
CHEMBL436 |
| Chemical and physical data | |
| Formula | C16H14F3NO3S |
| Molar mass | 357.34 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Tolrestat (INN) (AY-27773) is an aldose reductase inhibitor[1] which was approved for the control of certain diabetic complications.[2]
While it was approved for marketed in several countries, it failed a Phase III trial in the U.S. due to toxicity and never received FDA approval. It was discontinued by Wyeth in 1997 because of the risk of severe liver toxicity and death. It was sold under the tradename Alredase.
References
- ↑ Sestanj K, Bellini F, Fung S, et al. (March 1984). "N-[5-(trifluoromethyl)-6-methoxy-1-naphthalenyl]thioxomethyl]- N-methylglycine (Tolrestat), a potent, orally active aldose reductase inhibitor". J. Med. Chem. 27 (3): 255–6. doi:10.1021/jm00369a003. PMID 6422042.
- ↑ Kador PF, Kinoshita JH, Sharpless NE (July 1985). "Aldose reductase inhibitors: a potential new class of agents for the pharmacological control of certain diabetic complications". J. Med. Chem. 28 (7): 841–9. doi:10.1021/jm00145a001. PMID 3925146.
This article is issued from Wikipedia - version of the 11/16/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.