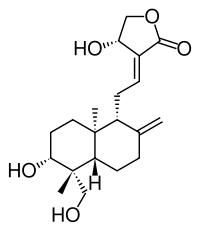
Andrographolide
 | |
| Names | |
|---|---|
| IUPAC name
3-[2-[Decahydro-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylene-1-napthalenyl]ethylidene]dihydro-4-hydroxy-2(3H)-furanone | |
| Identifiers | |
| 5508-58-7 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEBI | CHEBI:65408 |
| ChEMBL | ChEMBL186141 |
| ChemSpider | 16735664 |
| ECHA InfoCard | 100.024.411 |
| PubChem | 5318517 |
| UNII | 410105JHGR |
| |
| |
| Properties | |
| C20H30O5 | |
| Molar mass | 350.46 g·mol−1 |
| Appearance | Rhombic prisms or plates from ethanol or methanol |
| Density | 1.2317 g/cm3 |
| Melting point | 230 to 231 °C (446 to 448 °F; 503 to 504 K) |
| Sparingly soluble | |
| Related compounds | |
| Related labdanes |
14-deoxyandrographolide |
| Related compounds |
Xiyanping |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Andrographolide is a labdane diterpenoid that has been isolated from the stem and leaves of Andrographis paniculata.[1] Andrographolide is an extremely bitter substance.
Andrographolide has been studied for its effects on cell signaling, immunomodulation, and stroke.[2] Study has shown that andrographlide may bind to a spectrum of protein targets including NF-κB and actin by covalent modification.[3]
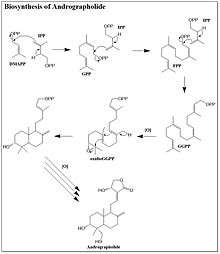
Biosynthesis
While Andrographolide is a relatively simple diterpene lactone, the biosynthesis by Andrographis paniculata was only recently determined. Andrographolide is a member of the isoprenoid family of natural products. The precursors to isoprenoid biosynthesis, isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), can be synthesized through either the mevalonic acid pathway (MVA) or deoxyxylulose pathway (DXP).[4] Through selective C13 labeling of the precursors to both the MVA and DXP pathways, it was determined that the majority of the andrographolide precursors are synthesized through the DXP pathway.[4] There are a small portion of andrographolide precursors synthesized through the MVA pathway. The biosynthesis of andrographolide begins with the addition of IPP to DMAPP, which forms geranyl pyrophosphate. Another molecule of IPP is then added, yielding farnesyl pyrophosphate (FPP). The final IPP molecule is added to the FPP to complete the backbone of the diterpene. The double bond originating from DMAPP is oxidized to an epoxide prior to the ring closing cascade that forms two six-membered rings. A series of oxidations form a five-membered lactone in addition to adding on the alcohol groups. The order of these post-synthetic modifications is not entirely known.[4]
References
- ↑ Chakravarti RN, Chakravarti D (1951). "Andrographolide, the active constituent of Andrographis paniculata Nees; a preliminary communication". Ind Med Gaz. 86 (3): 96–7. PMID 14860885.
- ↑ abcamBiochemicals Andrographolide-ab120636
- ↑ Wang, J; Tan, X. F.; Nguyen, V. S.; Yang, P; Zhou, J; Gao, M; Li, Z; Lim, T. K.; He, Y; Ong, C. S.; Lay, Y; Zhang, J; Zhu, G; Lai, S. L.; Ghosh, D; Mok, Y. K.; Shen, H. M.; Lin, Q (2014). "A quantitative chemical proteomics approach to profile the specific cellular targets of andrographolide, a promising anticancer agent that suppresses tumor metastasis". Molecular & Cellular Proteomics. 13 (3): 876–86. doi:10.1074/mcp.M113.029793. PMC 3945915
 . PMID 24445406.
. PMID 24445406. - 1 2 3 Srivastava, N; Akhila, A (2010). "Biosynthesis of andrographolide in Andrographis paniculata". Phytochemistry. 71 (11–12): 1298–304. doi:10.1016/j.phytochem.2010.05.022. PMID 20557910.