Isopentenyl pyrophosphate
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(hydroxy-(3-methylbut-3-enoxy) phosphoryl)oxyphosphonic acid | |
| Identifiers | |
| 358-71-4 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:128769 |
| ChemSpider | 13115335 |
| MeSH | isopentenyl+pyrophosphate |
| PubChem | 15983957 |
| |
| |
| Properties | |
| C5H12O7P2 | |
| Molar mass | 246.092 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
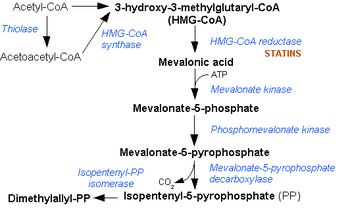
Isopentenyl pyrophosphate (IPP, isopentenyl diphosphate, or IDP)[1] is an intermediate in the classical, HMG-CoA reductase pathway (commonly called the mevalonate pathway), and is used by organisms in the biosynthesis of terpenes and terpenoids. IPP is formed from acetyl-CoA via the mevalonate pathway (the "upstream" part), and then is isomerized to dimethylallyl pyrophosphate by the enzyme isopentenyl pyrophosphate isomerase.[2]
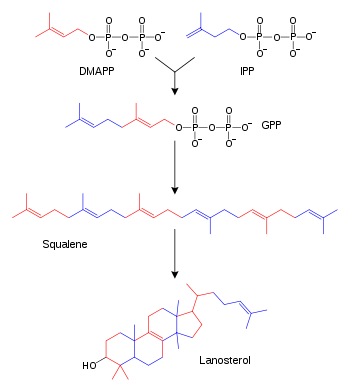
IPP can be synthesized via the alternative, non-mevalonate pathway of isoprenoid biosynthesis instead, where it is formed from (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) by the enzyme HMB-PP reductase (LytB, IspH). The non-mevalonate pathway is utilized by many bacteria, apicomplexan protozoa such as malaria parasites, and the plastids of higher plants.[3]
See also
References
- ↑ http://pubs.rsc.org/en/Content/ArticleLanding/2014/NP/C3NP70124G#!divAbstract
- ↑ Chang, Wei-chen; Song, Heng; Liu, Hung-wen; Liu, Pinghua "Current development in isoprenoid precursor biosynthesis and regulation" Current Opinion in Chemical Biology, 2013 volume 17, pp. 571-579. doi:10.1016/j.cbpa.2013.06.020
- ↑ Wiemer, AJ; Hsiao, CH; Wiemer, DF (2010). "Isoprenoid metabolism as a therapeutic target in gram-negative pathogens.". Current topics in medicinal chemistry. 10 (18): 1858–71. PMID 20615187.