Aspergillus fumigatus
| Aspergillus fumigatus | |
|---|---|
 | |
| Aspergillus fumigatus | |
| Scientific classification | |
| Kingdom: | Fungi |
| Phylum: | Ascomycota |
| Class: | Eurotiomycetes |
| Order: | Eurotiales |
| Family: | Trichocomaceae |
| Genus: | Aspergillus |
| Species: | fumigatus |
| Binomial name | |
| Aspergillus fumigatus Fresenius 1863 | |
| Synonyms | |
|
Neosartorya fumigata | |
Aspergillus fumigatus is a fungus of the genus Aspergillus, and is one of the most common Aspergillus species to cause disease in individuals with an immunodeficiency.
A. fumigatus, a saprotroph widespread in nature, is typically found in soil and decaying organic matter, such as compost heaps, where it plays an essential role in carbon and nitrogen recycling. Colonies of the fungus produce from conidiophores thousands of minute grey-green conidia (2–3 μm) that readily become airborne. For many years, A. fumigatus was thought to only reproduce asexually, as neither mating nor meiosis had ever been observed. In 2008, however, A. fumigatus was shown to possess a fully functional sexual reproductive cycle, 145 years after its original description by Fresenius.[1] Although A. fumigatus occurs in areas with widely different climates and environments, it displays low genetic variation and lack of population genetic differentiation on a global scale.[2] Thus the capability for sex is maintained even though little genetic variation is produced.
The fungus is capable of growth at 37 °C or 99 °F (normal human body temperature), and can grow at temperatures up to 50 °C or 122 °F, with conidia surviving at 70 °C or 158 °F—conditions it regularly encounters in self-heating compost heaps. Its spores are ubiquitous in the atmosphere, and it is estimated that everybody inhales several hundred spores each day; typically these are quickly eliminated by the immune system in healthy individuals. In immunocompromised individuals, such as organ transplant recipients and people with AIDS or leukemia, the fungus is more likely to become pathogenic, over-running the host's weakened defenses and causing a range of diseases generally termed aspergillosis. Several virulence factors have been postulated to explain this opportunistic behaviour.[3]
When the fermentation broth of A. fumigatus was screened, a number of indolic alkaloids with antimitotic properties were discovered.[4] The compounds of interest have been of a class known as tryprostatins, with spirotryprostatin B being of special interest as an anticancer drug.
A. fumigatus grown on certain building materials can produce genotoxic and cytotoxic mycotoxins, such as gliotoxin.[5]
Genome
A. fumigatus has a stable haploid genome of 29.4 million base pairs. The genome sequences of three Aspergillus species—Aspergillus fumigatus, Aspergillus nidulans, and Aspergillus oryzae—were published in the journal Nature in December 2005.[6][7][8]
Pathogenesis
A. fumigatus is the most frequent cause of invasive fungal infection in immunosuppressed individuals, which include patients receiving immunosuppressive therapy for autoimmune or neoplastic disease, organ transplant recipients, and AIDS patients.[9] A. fumigatus primarily causes invasive infection in the lung and represents a major cause of morbidity and mortality in these individuals.[10] Additionally, A. fumigatus can cause chronic pulmonary infections, allergic bronchopulmonary aspergillosis, or allergic disease in immunocompetent hosts.[11]
Innate immune response
Inhalational exposure to airborne conidia is continuous due to their ubiquitous distribution in the environment. However, in healthy individuals the innate immune system is an efficacious barrier to A. fumigatus infection.[11] A large portion of inhaled conidia are cleared by the mucociliary action of the respiratory epithelium.[11] Due to the small size of conidia, many conidia deposit in alveoli where they interact with epithelial and innate effector cells.[9][11] Alveolar macrophages phagocytize and destroy conidia within their phagosomes.[9][11] Epithelial cells, specifically type II pneumocytes, also internalize conidia which traffic to the lysosome where ingested conidia are destroyed.[9][11][12] First line immune cells also serve to recruit neutrophils and other inflammatory cells through release of cytokines and chemokines induced by ligation of specific fungal motifs to pathogen recognition receptors.[11] Neutrophils are essential for aspergillosis resistance, as demonstrated in neutropenic individuals, and are capable of sequestering both conidia and hyphae through distinct, non-phagocytic mechanisms.[9][10][11] Hyphae are too large for cell-mediated internalization, and thus neutrophil-mediated NADPH-oxidase induced damage represents the dominant host defense against hyphae.[9][11] In addition to these cell-mediated mechanisms of elimination, antimicrobial peptides secreted by the airway epithelium contribute to host defense.[9]
Invasion
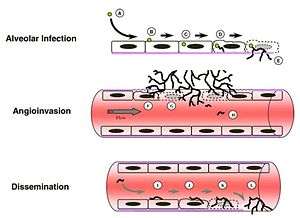
Immunosuppressed individuals are susceptible to invasive A. fumigatus infection, which most commonly manifests as invasive pulmonary aspergillosis. Inhaled conidia that evade host immune destruction are the progenitors of invasive disease. These conidia emerge from dormancy and make a morphological switch to hyphae by germinating in the warm, moist, nutrient-rich environment of the pulmonary alveoli.[9] Germination occurs both extracellularly or in type II pneumocyte endosomes containing conidia.[9][12] Following germination, filamentous hyphal growth results in epithelial penetration and subsequent penetration of the vascular endothelium.[9][12] The process of angioinvasion causes endothelial damage and induces a proinflammatory response, tissue factor expression and activation of the coagulation cascade.[9] This results in intravascular thrombosis and localized tissue infarction, however, dissemination of hyphal fragments is usually limited.[9][12] Dissemination through the blood stream only occurs in severely immunocomprimised individuals.[12]
Nutrient acquisition
A. fumigatus must acquire nutrients from its external environment in order to survive and flourish within its host. Many of the genes involved in such processes have been shown to impact virulence through experiments involving genetic mutation. Examples of nutrient uptake include that of metals, nitrogen, and macromolecules such as peptides.[10][13]
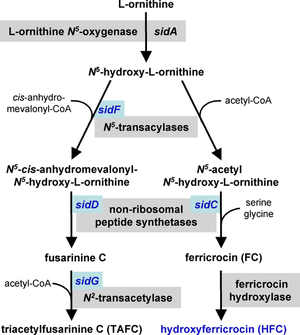
Iron acquisition
Iron is a necessary cofactor for many enzymes, and can act as a catalyst in the electron transport system. A. fumigatus has two mechanisms for the uptake of iron, reductive iron acquisition and siderophore-mediated.[15][16] Reductive iron acquisition includes conversion of iron from the ferric (Fe+3) to the ferrous (Fe+2) state and subsequent uptake via FtrA, an iron permease. Targeted mutation of the ftrA gene did not induce a decrease in virulence in the murine model of A. fumigatus invasion. In contrast, targeted mutation of sidA, the first gene in the sideophore biosynthesis pathway, proved sideophore-mediated iron uptake to be essential for virulence.[16][17] Mutation of the downstream sideophore biosynthesis genes sidC, sidD, sidF and sidG resulted in strains of A. fumigatus with similar decreases in virulence.[14] These mechanisms of iron uptake appear to work in parallel and both are upregulated in response to iron starvation.[16]
Nitrogen assimilation
A. fumigatus can survive on a variety of different nitrogen sources, and the assimilation of nitrogen is of clinical importance as it has been shown to affect virulence.[13][18] Proteins involved in nitrogen assimilation are transcriptionally regulated by the AfareA gene in A. fumigatus. Targeted mutation of the afareA gene showed a decrease in onset of mortality in a mouse model of invasion.[18] The Ras regulated protein RhbA has also been implicated in nitrogen assimilation. RhbA was found to be transcriptionally upregulated following contact of A. fumigatus with human endothelial cells, and strains with targeted mutation of the rhbA gene showed decreased growth on poor nitrogen sources and reduced virulence in vivo.[19]
Proteinases
The human lung contains large quantities of collagen and elastin, proteins that allow for tissue flexibility.[20] Aspergillus fumigatus produces and secretes elastases, proteases that cleave elastin in order to break down these macromolecular polymers for uptake. A significant correlation between the amount of elastase production and tissue invasion was first discovered in 1984.[21] Clinical isolates have also been found to have greater elastase activity than environmental strains of A. fumigatus.[22] A number of elastases have been characterized, including those from the serine protease, aspartic protease, and metalloprotease families.[23][24][25][26] Yet, the large redundancy of these elastases has hindered the identification of specific effects on virulence.[10][13]
Unfolded protein response
A number of studies who that the unfolded protein response (UPR) contributes to virulence of Aspergillus fumigatus.[27]
Secondary metabolism
Secondary metabolites in fungal development
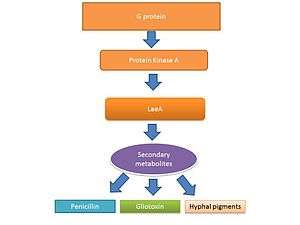
The life cycle of filamentous fungi including Aspergillus spp. consists of two phases: a hyphal growth phase and a reproductive (sporulation) phase. The switch between growth and reproductive phases of these fungi is regulated in part by the level of secondary metabolite production.[29][30] It is believed the secondary metabolites are produced to activate sporulation and pigments required for sporulation structures.[31][32] G protein signaling regulates secondary metabolite production.[33] Genome sequencing has revealed 40 potential genes involved in secondary metabolite production including mycotoxins, which are produced at the time of sporulation.[34][35]
Gliotoxin
Gliotoxin is a mycotoxin capable of altering host defenses through immunosuppression. Neutrophils are the principal targets of gliotoxin.[36][37] Gliotoxin interrupts the function of leukocytes by inhibiting migration and superoxide production and causes apoptosis in macrophages.[38] Gliotoxin disrupts the proinflammatory response through inhibition of NF-κB.[39]
Transcriptional regulation of gliotoxin
LaeA and GliZ are transcription factors known to regulate the production of gliotoxin. LaeA is a universal regulator of secondary metabolite production in Aspergillus spp.[28] LaeA influences the expression of 9.5% of the A. fumigatus genome, including many secondary metabolite biosynthesis genes such as nonribosomal peptide synthetases (NRPSs).[40] The production of numerous secondary metabolites, including gliotoxin, were impaired in an LaeA mutant (ΔlaeA) strain.[40] The ΔlaeA mutant showed increased susceptibility to macrophage phagocytosis and decreased ability to kill neutrophils ex vivo.[37] LaeA regulated toxins, besides gliotoxin, likely have a role in virulence since loss of gliotoxin production alone did not recapitulate the hypo-virulent ∆laeA pathotype.[40]
Gallery
-
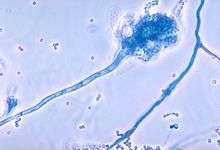
-

Conidia phialoconidia of Aspergillus fumigatus
-

Colony in Petri dish
-

Aspergillus fumigatus isolated from woodland soil
-
Slide of an infected turkey brain
See also
- 2012 US meningitis outbreak
- Allergic bronchopulmonary aspergillosis
- Aspergilloma
- Aspergillosis
- Diseases of the honeybee
- Fumagillin
- RodA
- Galactosaminogalactan
References
- ↑ O'Gorman CM; et al. (2008). "Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus". Nature. 457 (7228): 471–4. doi:10.1038/nature07528. PMID 19043401.
- ↑ Rydholm C, Szakacs G, Lutzoni F (April 2006). "Low genetic variation and no detectable population structure in Aspergillus fumigatus compared to closely related Neosartorya species". Eukaryotic Cell. 5 (4): 650–7. doi:10.1128/EC.5.4.650-657.2006. PMC 1459663
 . PMID 16607012.
. PMID 16607012. - ↑ Abad A, Fernández-Molina JV, Bikandi J, Ramírez A, Margareto J, Sendino J, Hernando FL, Pontón J, Garaizar J, Rementeria A (December 2010). "What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis." (PDF). Revista Iberoamericana de Micología. 27 (4): 155–182. doi:10.1016/j.riam.2010.10.003. PMID 20974273.
- ↑ Cui CB, et al. (1996). "Spirotryprostatin B, a novel mammalian cell cycle inhibitor produced by Aspergillus fumigatus". J. Antibiot. 49 (8): 832–835. doi:10.7164/antibiotics.49.832. PMID 8823522.
- ↑ Nieminen, SM; Kärki, R; Auriola, S; Toivola, M; Laatsch, H; Laatikainen, R; Hyvärinen, A; Von Wright, A (October 2002). "Isolation and Identification of Aspergillus fumigatus Mycotoxins on Growth Medium and Some Building Materials". Applied and Environmental Microbiology. 68 (10): 4871–5. doi:10.1128/aem.68.10.4871-4875.2002. PMC 126391
 . PMID 12324333
. PMID 12324333 - ↑ Galagan JE, et al. (2005). "Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae". Nature. 438 (7071): 1105–15. doi:10.1038/nature04341. PMID 16372000.
- ↑ Nierman WC, et al. (2005). "Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus". Nature. 438 (7071): 1151–6. doi:10.1038/nature04332. PMID 16372009.
- ↑ Machida M, et al. (2005). "Genome sequencing and analysis of Aspergillus oryzae". Nature. 438 (7071): 1157–61. doi:10.1038/nature04300. PMID 16372010.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Ben-Ami R, Lewis RE, Kontoyiannis DP (2010). "Enemy of the (immunosuppressed) state: an update on the pathogenesis of Aspergillus fumigatus infection". Br J Haematol. 150 (4): 406–17. doi:10.1111/j.1365-2141.2010.08283.x. PMID 20618330.
- 1 2 3 4 Hohl TM, Feldmesser M (2007). "Aspergillus fumigatus: Principles of Pathogenesis and Host Defense". Eukaryot Cell. 6 (11): 1953–63. doi:10.1128/EC.00274-07. PMC 2168400
 . PMID 17890370.
. PMID 17890370. - 1 2 3 4 5 6 7 8 9 Segal BH (2009). "Aspergillosis". N Engl J Med. 360 (18): 1870–84. doi:10.1056/NEJMra0808853. PMID 19403905.
- 1 2 3 4 5 6 Filler SG, Sheppard DC (2006). "Fungal Invasion of Normally Non-Phagocytic Host Cells". PLoS Pathog. 2 (12): e129. doi:10.1371/journal.ppat.0020129. PMC 1757199
 . PMID 17196036.
. PMID 17196036. - 1 2 3 Dagenais TR, Keller NP (2009). "Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis". Clin Microbiol Rev. 22 (3): 447–65. doi:10.1128/CMR.00055-08. PMC 2708386
 . PMID 19597008.
. PMID 19597008. - 1 2 Schrettl M, Bignell E, Kragl C, Sabiha Y, Loss O, Eisendle M, et al. (2007). "Distinct Roles for Intra- and Extracellular Siderophores during Aspergillus fumigatus Infection". PLoS Pathog. 3 (9): 1195–207. doi:10.1371/journal.ppat.0030128. PMC 1971116
 . PMID 17845073.
. PMID 17845073. - ↑ Haas H (2003). "Molecular genetics of fungal siderophore biosynthesis and uptake: the role of siderophores in iron uptake and storage". Appl Microbiol Biotechnol. 62 (4): 316–30. doi:10.1007/s00253-003-1335-2. PMID 12759789.
- 1 2 3 Schrettl M, Bignell E, Kragl C, Joechl C, Rogers T, Arst HN, et al. (2004). "Siderophore Biosynthesis But Not Reductive Iron Assimilation Is Essential for Aspergillus fumigatus Virulence". J Exp Med. 200 (9): 1213–9. doi:10.1084/jem.20041242. PMC 2211866
 . PMID 15504822.
. PMID 15504822. - ↑ Hissen AH, Wan AN, Warwas ML, Pinto LJ, Moore MM (2005). "The Aspergillus fumigatus Siderophore Biosynthetic Gene sidA, Encoding l-Ornithine N5-Oxygenase, Is Required for Virulence". Infect Immun. 73 (9): 5493–503. doi:10.1128/IAI.73.9.5493-5503.2005. PMC 1231119
 . PMID 16113265.
. PMID 16113265. - 1 2 Hensel M, Arst HN, Aufauvre-Brown A, Holden DW (1998). "The role of the Aspergillus fumigatus areA gene in invasive pulmonary aspergillosis". Mol Gen Genet. 258 (5): 553–7. doi:10.1007/s004380050767. PMID 9669338.
- ↑ Panepinto JC, Oliver BG, Amlung TW, Askew DS, Rhodes JC (2002). "Expression of the Aspergillus fumigatus rheb homologue, rhbA, is induced by nitrogen starvation". Fungal Genet Biol. 36 (3): 207–14. doi:10.1016/S1087-1845(02)00022-1. PMID 12135576.
- ↑ Rosenbloom J (1984). "Elastin: relation of protein and gene structure to disease". Lab Invest. 51 (6): 605–23. PMID 6150137.
- ↑ Kothary MH, Chase T, Macmillan JD (1984). "Correlation of elastase production by some strains of Aspergillus fumigatus with ability to cause pulmonary invasive aspergillosis in mice". Infect Immun. 43 (1): 320–5. PMC 263429
 . PMID 6360904.
. PMID 6360904. - ↑ Blanco JL, Hontecillas R, Bouza E, Blanco I, Pelaez T, Muñoz P, et al. (2002). "Correlation between the Elastase Activity Index and Invasiveness of Clinical Isolates of Aspergillus fumigatus". J Clin Microbiol. 40 (5): 1811–3. doi:10.1128/JCM.40.5.1811-1813.2002. PMC 130931
 . PMID 11980964.
. PMID 11980964. - ↑ Reichard U, Büttner S, Eiffert H, Staib F, Rüchel R (1990). "Purification and characterisation of an extracellular serine proteinase from Aspergillus fumigatus and its detection in tissue". J Med Microbiol. 33 (4): 243–51. doi:10.1099/00222615-33-4-243. PMID 2258912.
- ↑ Markaryan A, Morozova I, Yu H, Kolattukudy PE (1994). "Purification and characterization of an elastinolytic metalloprotease from Aspergillus fumigatus and immunoelectron microscopic evidence of secretion of this enzyme by the fungus invading the murine lung". Infect Immun. 62 (6): 2149–57. PMC 186491
 . PMID 8188335.
. PMID 8188335. - ↑ Lee JD, Kolattukudy PE (1995). "Molecular cloning of the cDNA and gene for an elastinolytic aspartic proteinase from Aspergillus fumigatus and evidence of its secretion by the fungus during invasion of the host lung". Infect Immun. 63 (10): 3796–803. PMC 173533
 . PMID 7558282.
. PMID 7558282. - ↑ Iadarola P, Lungarella G, Martorana PA, Viglio S, Guglielminetti M, Korzus E, et al. (1998). "Lung injury and degradation of extracellular matrix components by Aspergillus fumigatus serine proteinase". Exp Lung Res. 24 (3): 233–51. doi:10.3109/01902149809041532. PMID 9635248.
- ↑ Feng, Xizhi; Krishnan, Karthik; Richie, Daryl L.; Aimanianda, Vishukumar; Hartl, Lukas; Grahl, Nora; Powers-Fletcher, Margaret V.; Zhang, Minlu; Fuller, Kevin K. (2011-10-01). "HacA-independent functions of the ER stress sensor IreA synergize with the canonical UPR to influence virulence traits in Aspergillus fumigatus". PLoS Pathog. 7 (10): e1002330. doi:10.1371/journal.ppat.1002330. ISSN 1553-7374. PMC 3197630
 . PMID 22028661.
. PMID 22028661. - 1 2 Bok JW, Keller NP (2004). "LaeA, a Regulator of Secondary Metabolism in Aspergillus spp". Eukaryot Cell. 3 (2): 527–35. doi:10.1128/EC.3.2.527-535.2004. PMC 387652
 . PMID 15075281.
. PMID 15075281. - ↑ Calvo AM, Wilson RA, Bok JW, Keller NP (2002). "Relationship between Secondary Metabolism and Fungal Development". Microbiol Mol Biol Rev. 66 (3): 447–59, table of contents. doi:10.1128/MMBR.66.3.447-459.2002. PMC 120793
 . PMID 12208999.
. PMID 12208999. - ↑ Tao L, Yu JH (2011). "AbaA and WetA govern distinct stages of Aspergillus fumigatus development". Microbiology. 157 (Pt 2): 313–26. doi:10.1099/mic.0.044271-0. PMID 20966095.
- ↑ Adams TH, Yu JH (1998). "Coordinate control of secondary metabolite production and asexual sporulation in Aspergillus nidulans". Curr Opin Microbiol. 1 (6): 674–7. doi:10.1016/S1369-5274(98)80114-8. PMID 10066549.
- ↑ Kawamura C, Tsujimoto T, Tsuge T (1999). "Targeted disruption of a melanin biosynthesis gene affects conidial development and UV tolerance in the Japanese pear pathotype of Alternaria alternata". Mol Plant Microbe Interact. 12 (1): 59–63. doi:10.1094/MPMI.1999.12.1.59. PMID 9885194.
- ↑ Brodhagen M, Keller NP (2006). "Signalling pathways connecting mycotoxin production and sporulation". Mol Plant Pathol. 7 (4): 285–301. doi:10.1111/j.1364-3703.2006.00338.x. PMID 20507448.
- ↑ Nierman WC, Pain A, Anderson MJ, Wortman JR, Kim HS, Arroyo J, et al. (2005). "Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus". Nature. 438 (7071): 1151–6. doi:10.1038/nature04332. PMID 16372009.
- ↑ Trail F, Mahanti N, Linz J (1995). "Molecular biology of aflatoxin biosynthesis". Microbiology. 141 (4): 755–65. doi:10.1099/13500872-141-4-755. PMID 7773383.
- ↑ Spikes S, Xu R, Nguyen CK, Chamilos G, Kontoyiannis DP, Jacobson RH, et al. (2008). "Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence". J Infect Dis. 197 (3): 479–86. doi:10.1086/525044. PMID 18199036.
- 1 2 Bok JW, Chung D, Balajee SA, Marr KA, Andes D, Nielsen KF, et al. (2006). "GliZ, a Transcriptional Regulator of Gliotoxin Biosynthesis, Contributes to Aspergillus fumigatus Virulence". Infect Immun. 74 (12): 6761–8. doi:10.1128/IAI.00780-06. PMC 1698057
 . PMID 17030582.
. PMID 17030582. - ↑ Kamei K, Watanabe A (2005). "Aspergillus mycotoxins and their effect on the host". Med Mycol. 43 Suppl 1: S95–9. PMID 16110799.
- ↑ Gardiner DM, Waring P, Howlett BJ (2005). "The epipolythiodioxopiperazine (ETP) class of fungal toxins: distribution, mode of action, functions and biosynthesis". Microbiology. 151 (Pt 4): 1021–32. doi:10.1099/mic.0.27847-0. PMID 15817772.
- 1 2 3 Perrin RM, Fedorova ND, Bok JW, Cramer RA, Wortman JR, Kim HS, et al. (2007). "Transcriptional Regulation of Chemical Diversity in Aspergillus fumigatus by LaeA". PLoS Pathog. 3 (4): e50. doi:10.1371/journal.ppat.0030050. PMC 1851976
 . PMID 17432932.
. PMID 17432932.
External links
| Wikimedia Commons has media related to Aspergillus fumigatus. |
- Emergence of Azole Resistance in Aspergillus fumigatus and Spread of a Single Resistance Mechanism. at SciVee
- The Aspergillus Trust A registered UK charity engaged in support of sufferers of aspergillus disease worldwide and research into cures
- The Fungal Research Trust
- Aspergillus info from DoctorFungus.org