Borabenzene
A borabenzene is a heteroaromatic compound that has a boron atom instead of the carbon atom of a benzene molecule. A free borabenzene, which has no donor ligand on the boron atom, has not yet been isolated despite its simple structure and the chemical robustness of boron-carbon bonds. The instability of this molecule results from the high Lewis acidity of the boron atom due to its electron deficiency. All attempts to create a free borabenzene have resulted in formations of complexes with anionic or neutral ligands (even a nitrogen molecule can coordinate to a borabenzene). However, the complexes with anionic ligands, called boratabenzenes, show rich chemistry including the coordination chemistry as anionic π-type ligands. Borabenzene also forms stable adducts with electron donor molecules such as pyridine (the adduct with which is isoelectronic with biphenyl) or triphenylphosphine (the adduct with which is isoelectronic with tetraphenylsilane).
Properties as ligands
Boratabenzenes have a negative charge on their six-membered rings and act as strong π-donating ligands like a cyclopentadienyl anion, which is often used in transition metal complexes. In fact, sandwich or half-sandwich type complexes of many transition metals have been reported using boratabenzenes, and the zirconium sandwich complexes exhibit catalytic activity in ethylene polymerization similar to zirconocenes, which are the cyclopentadienyl counterparts.
Family of boratabenzenes
Several boracyclic compounds are involved in the family of boratabenzenes: 1-boratanaphthalene, 9-borathaanthracene, boracyclooctatetraene, and 2,2’-diboratabiphenyl. Among these compounds, 2,2’-diboratabiphenyl is the first bidentate Lewis acid based on a borabenzene framework, so it is regarded as a Lewis acid analogue of 2,2’-bipyridine, a common bidentate ligand. This compound forms polycyclic aromatic hydrocarbon analogues containing boron and nitrogen atoms, which show characteristic optical and electrochemical properties due to the charge transfer between the boron and nitrogen atoms.
Reactions
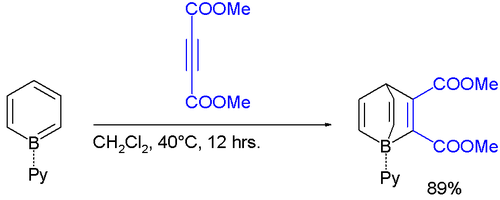
Borabenzene is known to react with electron-deficient alkynes in a Diels-Alder reaction to the boron pendants of barrelene called borabarrelenes:[1]
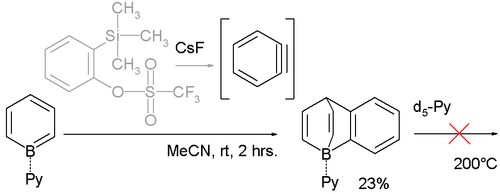
Likewise the benzoborabarrelene is accessible through reaction with benzyne through 2-(Trimethylsilyl)phenyl triflate and caesium fluoride:
The boron atom in these barrelene compounds is pyramidalized (the sum of the C-B-C angles is 311° not 327°) leading to even greater Lewis acid strength. This is evident from the unusual small B-N bond length of 158 picometer compared to 165pm for ordinary B-N bonds in borane-pyridine adducts.
Another remarkable manifestation of this property is their reluctance to surrender the pyridine ligand (for example in exchange for deuterated pyridine d5-Py), even at 200 °C.
Unlike ordinary barrelenes, this benzobarrelene is also resistant to ring-opening metathesis polymerisation.
See also
- 6-membered aromatic rings with one carbon replaced by another group: borabenzene, benzene, silabenzene, germabenzene, stannabenzene, pyridine, phosphorine, arsabenzene, pyrylium salt
- Borazine
References
- ↑ 1-Borabarrelene Derivatives via Diels-Alder Additions to Borabenzenes Thomas K. Wood, Warren E. Piers, Brian A. Keay, and Masood Parvez Org. Lett.; 2006; 8(13) pp 2875 - 2878; (Letter) doi:10.1021/ol061201w