Carbanion
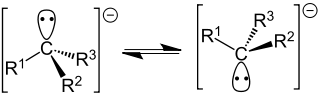
A carbanion is an anion in which carbon is tervalent (forms three bonds) and bears a formal negative charge in at least one significant mesomeric contributor (resonance form).[1] Absent π delocalization, carbanions assume a trigonal pyramidal, bent, or linear geometry when the carbanionic carbon is bound to three (e.g., methyl anion), two (e.g., phenyl anion), or one (e.g., acetylide anion) substituents, respectively. Formally, a carbanion is the conjugate base of a carbon acid:
- R3C-H + B− → R3C− + H-B
where B stands for the base. A carbanion is one of several reactive intermediates in organic chemistry. In organic synthesis, organolithium reagents and Grignard reagents are commonly regarded as carbanions. This is a convenient approximation, although these species are almost always multinuclear clusters containing polar covalent bonds rather than true carbanions.
Trends and occurrence
Carbanions are typically nucleophile and basic. The basicity and nucleophilicity of carbanions are determined by the substituents on carbon. These include
- The inductive effect. Electronegative atoms adjacent to the charge will stabilize the charge;
- The extent of conjugation of the anion. Resonance effects can stabilize the anion. This is especially true when the anion is stabilized as a result of aromaticity.
Geometry also affects the orbital hybridization of the charge-bearing carbanion. The greater the s-character of the charge-bearing atom, the more stable the anion.
A carbanion are often reactive intermediates in organic chemistry. For instance in the E1cB elimination reaction and in organometallic chemistry in for instance a Grignard reaction or in alkyl lithium chemistry. Stable carbanions do however exist. In 1984 Olmstead presented the lithium crown ether salt of the triphenylmethyl carbanion from triphenylmethane, n-butyllithium and 12-crown-4 at low temperatures:[2]
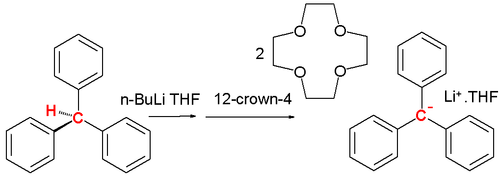
Adding n-butyllithium to triphenylmethane in THF at low temperatures followed by 12-crown-4 results in a red solution and the salt complex precipitates at −20 °C. The central C-C bond lengths are 145 pm with the phenyl ring propelled at an average angle of 31.2°. This propeller shape is less pronounced with a tetramethylammonium counterion .[3]
One tool for the detection of carbanions in solution is proton NMR.[4] A spectrum of cyclopentadiene in DMSO shows four vinylic protons at 6.5 ppm and two methylene bridge protons at 3 ppm whereas the cyclopentadienyl anion has a single resonance at 5.50 ppm.
Carbon acids
Any compound containing hydrogen can, in principle, undergo deprotonation to form its conjugate base. A compound is a carbon acid if deprotonation results in loss of a proton from a carbon atom. Compared to compounds typically considered to be acids (e.g., mineral acids like nitric acid, or carboxylic acids like acetic acid), carbon acids are typically many orders of magnitude weaker, although exceptions exist (see below). For example, benzene is not an acid in the classical Arrhenius sense, since its aqueous solutions are neutral. Nevertheless, it is very weak Brønsted acid with an estimated pKa of 49 which may undergo deprotonation in the presence of a superbase like the Lochmann-Schlosser base (n-BuLi:KOt-Bu). As conjugate acid-base pairs, the factors that determine the relative stability of carbanions also determine the ordering of the pKa values of the corresponding carbon acids. Furthermore, pKa values allow the prediction of whether a proton transfer process will be thermodynamically favorable: In order for the deprotonation of an acidic species HA with base B− to be thermodynamically favorable (K > 1), the relationship pKa(BH) > pKa(AH) must hold.
These values below are pKa values determined in DMSO, which has a broader useful range (~0 to ~35) than values determined in water (~0 to ~15) and better reflect the basicity of the carbanions in typical organic solvents. Values below less than 0 or greater than 35 are indirectly estimated; hence, the numerical accuracy of these values is limited. Aqueous pKa values are also commonly encountered in the literature, particularly in the context of biochemistry and enzymology. Moreover, aqueous values are often given in introductory organic chemistry textbooks for pedagogical reasons, although the issue of solvent dependence is often glossed over. In general, pKa values in water and organic solvent diverge significantly when the anion is capable of hydrogen bonding. For instance, in the case of water, the values differ dramatically: pKaaq(H2O) = 15.7, while pKaDMSO(H2O) = 31.4,[5] reflecting the differing ability of water and DMSO to stabilize hydroxide anion. On the other hand, for cyclopentadiene, the numerical values are comparable: pKaaq(Cp-H) = 15, while pKaDMSO(Cp-H) = 18.[5]
| name | formula | structural formula | pKaDMSO |
|---|---|---|---|
| Cyclopentane | C5H10 | ~ 59 | |
| Methane | CH4 |  |
~ 56 |
| Benzene | C6H6 | ~ 49[6] | |
| Propene | C3H6 | ~ 44 | |
| Toluene | C6H5CH3 | ~ 43 | |
| Ammonia (N-H) | NH3 |  |
~ 41 |
| Dithiane | C4H8S2 | 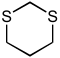 |
~ 39 |
| Dimethyl sulfoxide | (CH3)2SO |  |
35.1 |
| Diphenylmethane | C13H12 | 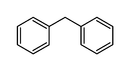 |
32.3 |
| Acetonitrile | CH3CN | 31.3 | |
| Aniline (N-H) | C6H5NH2 |  |
30.6 |
| Triphenylmethane | C19H16 |  |
30.6 |
| Fluoroform | CHF3 |  |
30.5 |
| Xanthene | C13H10O | 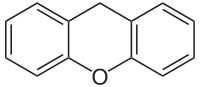 |
30.0 |
| Ethanol (O-H) | C2H5OH | 29.8 | |
| Phenylacetylene | C8H6 |  |
28.8 |
| Thioxanthene | C13H10S | 28.6 | |
| Acetone | C3H6O | 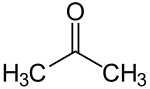 |
26.5 |
| Benzoxazole | C7H5NO | 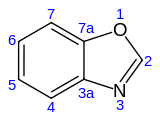 |
24.4 |
| Fluorene | C13H10 | 22.6 | |
| Indene | C9H8 | 20.1 | |
| Cyclopentadiene | C5H6 | 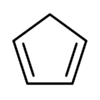 |
18.0 |
| Nitromethane | CH3NO2 | 17.2 | |
| Diethyl malonate | C7H12O4 |  |
16.4 |
| Acetylacetone | C5H8O2 | 13.3 | |
| Hydrogen cyanide | HCN | 12.9 | |
| Acetic acid (O-H) | CH3COOH |  |
12.6 |
| Malononitrile | C3H2N2 |  |
11.1 |
| Dimedone | C8H12O2 | 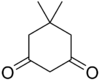 |
10.3 |
| Meldrum's acid | C6H8O4 | 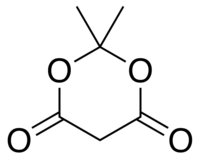 |
7.3 |
| Hydrogen chloride (Cl-H) | HCl | HCl (g) | –2.0[7] |
| Triflidic acid | HC(SO2CF3)3 | ~ –16[8] | |
| Table 1. Carbon acid acidities in pKa in DMSO.[9] These values may differ significantly from aqueous pKa values. | |||
Note that acetic acid, ammonia, aniline, ethanol, and hydrogen chloride are not carbon acids, but are common acids shown for comparison.
As indicated by the examples above, acidity increases (pKa decreases) when the negative charge is delocalized. This effect occurs when the substituents on the carbanion are unsaturated or electronegative.
The acidity of the α-hydrogen in carbonyl compounds crucially these compounds to participate in synthetically important C–C bond-forming reactions including the aldol reaction and Michael addition.
Chiral carbanions
With the molecular geometry for a carbanion described as a trigonal pyramid the question is whether or not carbanions can display chirality, because if the activation barrier for inversion of this geometry is too low any attempt at introducing chirality will end in racemization, similar to the nitrogen inversion. However, solid evidence exists that carbanions can indeed be chiral for example in research carried out with certain organolithium compounds.
The first ever evidence for the existence of chiral organolithium compounds was obtained in 1950. Reaction of chiral 2-iodooctane with sec-butyllithium in petroleum ether at −70 °C followed by reaction with dry ice yielded mostly racemic 2-methylbutyric acid but also an amount of optically active 2-methyloctanoic acid which could only have formed from likewise optical active 2-methylheptyllithium with the carbon atom linked to lithium the carbanion:[10]
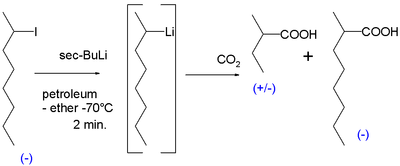
On heating the reaction to 0 °C the optical activity is lost. More evidence followed in the 1960s. A reaction of the cis isomer of 2-methylcyclopropyl bromide with sec-butyllithium again followed by carboxylation with dry ice yielded cis-2-methylcyclopropylcarboxylic acid. The formation of the trans isomer would have indicated that the intermediate carbanion was unstable.[11]

In the same manner the reaction of (+)-(S)-l-bromo-l-methyl-2,2-diphenylcyclopropane with n-butyllithium followed by quench with methanol resulted in product with retention of configuration:[12]
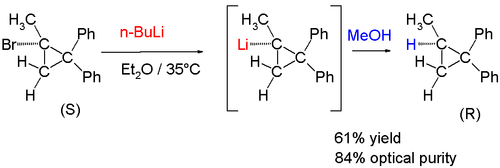
Of recent date are chiral methyllithium compounds:[13]
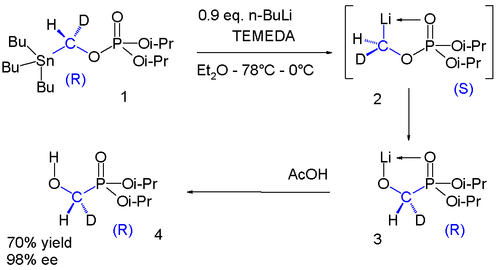
The phosphate 1 contains a chiral group with a hydrogen and a deuterium substituent. The stannyl group is replaced by lithium to intermediate 2 which undergoes a phosphate-phosphorane rearrangement to phosphorane 3 which on reaction with acetic acid gives alcohol 4. Once again in the range of −78 °C to 0 °C the chirality is preserved in this reaction sequence.[14]
History
A carbanionic structure first made an appearance in the reaction mechanism for the benzoin condensation as correctly proposed by Clarke and Lapworth in 1907.[15] In 1904 Schlenk prepared Ph3C−NMe4+ in a quest for pentavalent nitrogen (from tetramethylammonium chloride and Ph3CNa) [16] and in 1914 he demonstrated how triarylmethyl radicals could be reduced to carbonions by alkali metals [17] The phrase carbanion was introduced by Wallis and Adams in 1933 as the negatively charged counterpart of the carbonium ion [18][19]
External links
- Large database of Bordwell pKa values at www.chem.wisc.edu Link
- Large database of Bordwell pKa values at daecr1.harvard.edu Link
See also
References
- ↑ "IUPAC Gold Book - carbanion". goldbook.iupac.org. Retrieved 2016-07-30.
- ↑ The isolation and X-ray structures of lithium crown ether salts of the free phenyl carbanions [CHPh2]- and [CPh3]- Marilyn M. Olmstead, Philip P. Power; J. Am. Chem. Soc.; 1985; 107(7); 2174-2175. doi:10.1021/ja00293a059
- ↑ Harder, S. (2002). "Schlenk's Early "Free" Carbanions". Chemistry: A European Journal. 8 (14): 3229–3229. doi:10.1002/1521-3765(20020715)8:14<3229::AID-CHEM3229>3.0.CO;2-3.
- ↑ A Simple and Convenient Method for Generation and NMR Observation of Stable Carbanions. Hamid S. Kasmai Journal of Chemical Education • Vol. 76 No. 6 June 1999
- 1 2 Evans, D. A.; Ripin, D. H. (2005). "Chem 206 pKa Table" (PDF).
- ↑ Bordwell, G. F.; Matthews, Walter S. (2002-05-01). "Equilibrium acidities of carbon acids. III. Carbon acids in the membrane series". Journal of the American Chemical Society. 96 (4): 1216–1217. doi:10.1021/ja00811a041.
- ↑ Trummal, Aleksander; Lipping, Lauri; Kaljurand, Ivari; Koppel, Ilmar A.; Leito, Ivo (2016-05-06). "Acidity of Strong Acids in Water and Dimethyl Sulfoxide". The Journal of Physical Chemistry A. 120 (20): 3663–3669. doi:10.1021/acs.jpca.6b02253.
- ↑ The reported pKa in MeCN is –3.7 (J. Org. Chem. 2011, 76, 391). The pKa in DMSO was estimated by the correlation pKaMeCN = 0.98 × pKaDMSO + 11.6 (J. Org. Chem. 2009, 74, 2679).
- ↑ Equilibrium acidities in dimethyl sulfoxide solution Frederick G. Bordwell Acc. Chem. Res.; 1988; 21(12) pp 456 - 463; doi:10.1021/ar00156a004
- ↑ FORMATION OF OPTICALLY ACTIVE 1-METHYLHEPTYLLITHIUM Robert L. Letsinger J. Am. Chem. Soc.; 1950; 72(10) pp 4842 - 4842; doi:10.1021/ja01166a538
- ↑ The Configurational Stability of cis- and trans-2-Methylcyclopropyllithium and Some Observations on the Stereochemistry of their Reactions with Bromine and Carbon Dioxide Douglas E. Applequist and Alan H. Peterson J. Am. Chem. Soc.; 1961; 83(4) pp 862 - 865; doi:10.1021/ja01465a030
- ↑ Cyclopropanes. XV. The Optical Stability of 1-Methyl-2,2-diphenylcyclopropyllithium H. M. Walborsky, F. J. Impastato, and A. E. Young J. Am. Chem. Soc.; 1964; 86(16) pp 3283 - 3288; doi:10.1021/ja01070a017
- ↑ Preparation of Chiral -Oxy-[2H1]methyllithiums of 99% ee and Determination of Their Configurational Stability Dagmar Kapeller, Roland Barth, Kurt Mereiter, and Friedrich Hammerschmidt J. Am. Chem. Soc.; 2007; 129(4) pp 914 - 923; (Article) doi:10.1021/ja066183s
- ↑ Enantioselectivity determined by NMR spectroscopy after derivatization with Mosher's acid
- ↑ Clarke, R. W. L.; Lapworth, A. (1907). "LXV.?An extension of the benzoin synthesis". Journal of the Chemical Society, Transactions. 91: 694. doi:10.1039/CT9079100694.
- ↑ Schlenk, W.; Weickel, T.; Herzenstein, A. (1910). "Ueber Triphenylmethyl und Analoga des Triphenylmethyls in der Biphenylreihe. [Zweite Mittheilung über „Triarylmethyle".]". Justus Liebig's Annalen der Chemie. 372: 1. doi:10.1002/jlac.19103720102.
- ↑ Schlenk, W.; Marcus, E. (1914). "Über Metalladditinen an freie organische Radikale. (Über Triarylmethyle. XII.)". Berichte der deutschen chemischen Gesellschaft. 47 (2): 1664. doi:10.1002/cber.19140470256.
- ↑ Wallis, E. S.; Adams, F. H. (1933). "The Spatial Configuration of the Valences in Tricovalent Carbon Compounds1". Journal of the American Chemical Society. 55 (9): 3838. doi:10.1021/ja01336a068.
- ↑ Tidwell, T. T. (1997). "The first century of physical organic chemistry: A prologue". Pure and Applied Chemistry. 69 (2): 211–214. doi:10.1351/pac199769020211.