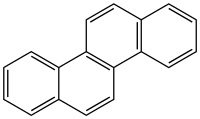
Chrysene
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Chrysene | |
| Other names
Benzo[a]phenanthrene 1,2-Benzphenanthrene | |
| Identifiers | |
| 218-01-9 | |
| 3D model (Jmol) | Interactive image |
| 1909297 | |
| ChEBI | CHEBI:51687 |
| ChEMBL | ChEMBL85685 |
| ChemSpider | 8817 |
| ECHA InfoCard | 100.005.386 |
| EC Number | 205-923-4 |
| 262600 | |
| KEGG | C14222 |
| PubChem | 10457109 |
| RTECS number | GC0700000 |
| UNII | 084HCM49PT |
| |
| |
| Properties | |
| C18H12 | |
| Molar mass | 228.29 g·mol−1 |
| Appearance | Orthorhombic bipyramidal plates |
| Density | 1.274 g/cm3 |
| Melting point | 254 °C (489 °F; 527 K) |
| Boiling point | 448 °C (838 °F; 721 K) |
| Insoluble | |
| Solubility in ethanol | 1 g/1300 mL[2] |
| Related compounds | |
| Related PAHs |
Pyrene, Tetracene, Triphenylene |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Chrysene is a polycyclic aromatic hydrocarbon (PAH) with the molecular formula C
18H
12[3] that consists of four fused benzene rings. It is a natural constituent of coal tar, from which it was first isolated and characterized. It is also found in creosote at levels of 0.5-6 mg/kg.[4]
The name "chrysene" originates from Greek Χρύσoς (chrysos), meaning "gold", and is due to the golden-yellow color of the crystals of the hydrocarbon, thought to be the proper color of the compound at the time of its isolation and characterization. However, high purity chrysene is colorless, the yellow hue being due to the traces of its yellow-orange isomer tetracene, which cannot be separated easily.
Occurrence
More than 20% of the carbon in the universe may be associated with PAHs, which have been implicated as starting materials for the formation of life. PAHs seem to have been formed shortly after the Big Bang, are widespread throughout the universe, and are associated with new stars and exoplanets.[5][6]
Safety
As with other PAHs, chrysene is suspected to be a human carcinogen. Some evidence suggests that it cause cancer in laboratory animals,[7] but chrysene are often contaminated with more strongly carcinogenic compounds. Chrysene is estimated to be about 1% of the toxicity of benzopyrene.[8]
Derivatives
Derivatives of chrysene include tetrahydrochrysene and 2,8-dihydroxyhexahydrochrysene, which are estrogenic compounds. The experimental cancer drug crisnatol is a derivative of chrysene.
See also
References
- ↑ Merck Index, 11th Edition, 2259.
- ↑ Merck Index, 14th edition
- ↑
 Chisholm, Hugh, ed. (1911). "Chrysene". Encyclopædia Britannica. 6 (11th ed.). Cambridge University Press. p. 319.
Chisholm, Hugh, ed. (1911). "Chrysene". Encyclopædia Britannica. 6 (11th ed.). Cambridge University Press. p. 319. - ↑ Anja Sörensen and Bodo Wichert "Asphalt and Bitumen" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, Weinheim, 2009. doi:10.1002/14356007.a03_169.pub2http://www.qrpoil.com/site/?bitumen
- ↑ Hoover, Rachel (February 21, 2014). "Need to Track Organic Nano-Particles Across the Universe? NASA's Got an App for That". NASA. Retrieved February 22, 2014.
- ↑ NASA Ames PAH IR Spectroscopic Database
- ↑ TOXICOLOGICAL PROFILE FOR POLYCYCLIC AROMATIC HYDROCARBONS
- ↑ Ian C.T. Nisbet, Peter K. LaGoy "Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs)" Regulatory Toxicology and Pharmacology 1992, Volume 16, Pages 290-300. doi:10.1016/0273-2300(92)90009-X