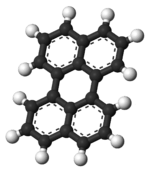
Perylene
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Perylene | |||
| Other names
peri-Dinaphthalene; Perilene; Dibenz[de,kl]anthracene | |||
| Identifiers | |||
| 198-55-0 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEBI | CHEBI:29861 | ||
| ChEMBL | ChEMBL1797415 | ||
| ChemSpider | 8788 | ||
| ECHA InfoCard | 100.005.365 | ||
| KEGG | C19497 | ||
| PubChem | 9142 | ||
| UNII | 5QD5427UN7 | ||
| |||
| |||
| Properties | |||
| C20H12 | |||
| Molar mass | 252.32 g·mol−1 | ||
| Appearance | Brown solid | ||
| Melting point | 276 to 279 °C (529 to 534 °F; 549 to 552 K) | ||
| Hazards | |||
| S-phrases | S22 S24/25 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Perylene or perilene is a polycyclic aromatic hydrocarbon with the chemical formula C20H12, occurring as a brown solid. It or its derivatives may be carcinogenic, and it is considered to be a hazardous pollutant. In cell membrane cytochemistry, perylene is used as a fluorescent lipid probe. It is the parent compound of a class of rylene dyes.
Emission
Perylene displays blue fluorescence. It is used as a blue-emitting dopant material in OLEDs, either pure or substituted. Perylene can be also used as an organic photoconductor. It has an absorption maximum at 434 nm, and as with all polycyclic aromatic compounds, low water solubility (1.2 x 10−5 mmol/L). Perylene has a molar absorptivity of 38,500 M−1cm−1 at 435.7 nm.
 Perylene dissolved in dichloromethane exposed to Long Wave UV radiation
Perylene dissolved in dichloromethane exposed to Long Wave UV radiation Perylene dissolved in dichloromethane exposed to Short Wave UV radiation
Perylene dissolved in dichloromethane exposed to Short Wave UV radiation
Structure
The perylene molecule consists of two naphthalene molecules connected by a carbon-carbon bond at the 1 and 8 positions on both molecules. All of the carbon atoms in perylene are sp2 hybridized. The structure of perylene has been extensively studied by X-ray crystallography.[2]
References
- ↑ Perylene at Sigma-Aldrich
- ↑ Donaldson, D. M.; Robertson, J. M.; White, J. G. (1953). "The crystal and molecular structure of perylene". Proceedings of the Royal Society A. 220 (1142): 311–321. doi:10.1098/rspa.1953.0189. JSTOR 99329.