Cobalamin riboswitch
| Cobalamin riboswitch | |
|---|---|
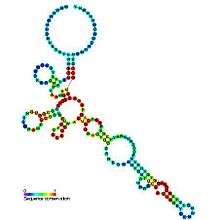 | |
| Predicted secondary structure and sequence conservation of Cobalamin | |
| Identifiers | |
| Symbol | Cobalamin |
| Rfam | RF00174 |
| Other data | |
| RNA type | Cis-reg; riboswitch |
| Domain(s) | Bacteria |
| SO | 0000035 |
Cobalamin riboswitch is a cis-regulatory element which is widely distributed in 5' untranslated regions of vitamin B12 (Colbalamin) related genes in bacteria.[1] Riboswitches are metabolite binding domains within certain messenger RNAs (mRNAs) that serve as precision sensors for their corresponding targets. Allosteric rearrangement of mRNA structure is mediated by ligand binding, and this results in modulation of gene expression or translation of mRNA to yield a protein. Cobalamin in the form of adenosylcobalamin (Ado-CBL) is known to repress expression of proteins for vitamin B12 biosynthesis via a post-transcriptional regulatory mechanism that involves direct binding of Ado-CBL to 5' UTRs in relevant genes, preventing ribosome binding and translation of those genes. Before proof of riboswitch function, a conserved sequence motif called the B12 box[2] was identified that corresponds to a part of the cobalamin riboswitch,[1] and a more complete conserved structure was identified.[3][4] Variants of the riboswitch consensus have been identified.[5]
AdoCbl riboswitch
| AdoCbl riboswitch | |
|---|---|
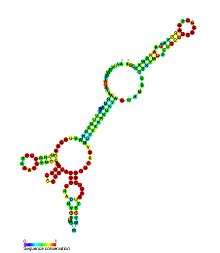 | |
| Predicted secondary structure and sequence conservation of AdoCbl riboswitch | |
| Identifiers | |
| Symbol | AdoCbl_riboswitch |
| Alt. Symbols | rli55 |
| Rfam | RF01482 |
| Other data | |
| RNA type | Cis-reg; riboswitch; |
| Domain(s) | Bacteria; |
| GO | 0061020 0046336 |
| SO | 0005836 0000035 |
AdoCbl riboswitch is a cis-regulatory riboswitch element present in untranslated regions of mRNA where binding adenosylcobalamin (AdoCbl) causes a change in gene expression. This riboswitch has been shown to be conserved across several strains of bacteria and has an influence over the expression of the ethanolamine utilization genes. It is proposed to be a subclass of the previously described classical AdoCbl-sensing riboswitch.[6]
Ethanolamine is abundant in the human intestinal tract as it is the product of the breakdown of the phosphatidylethanolamine from cell membranes and is also present in processed food. Most bacteria that inhabit the intestinal tract can utilize ethanolamine as a carbon and nitrogen source by upregulating the expression of the ethanolamine utilization genes; This may have a survival advantage.[7]
The expression of the ethanolamine utilization genes (eutG) is influenced by two different mechanisms. The first is a two component regulatory system that senses the presence of ethanolamine and the second mechanism is an AdoCbl riboswitch that senses the presence of AdoCbl, a cofactor needed for the breakdown of ethanolamine. A study showed that both these regulatory elements need to be activated for the bacteria to grow efficiently on medium containing ethanolamine.[8] Bioinformatic studies were initially unsuccessful in identifying AdoCbl riboswitches within the bacteria genomes, but subsequent studies of the intergenic regions of the eutG locus using Ribex identified an RNA element between the eutT and eutG genes.[1][9]
This new subclass of AdoCbl riboswitches has both sequence and structural conservation with the core region of the classical AdoCbl-sensing riboswitches however it has some clear differences. The new element lacks a paired region formed by the pairing of the 5'3' ends found in canonical AdoCbl riboswitches. It also has an additional extra base-paired region near the 3′ terminus that is absent from the classical AdoCbl riboswitches.[6]
In line probing analysis of this new riboswitch showed that the presence of AdoCbl increased mRNA stability and causes a global conformational change in the riboswitch.[6] This riboswitch unlike previously characterized AdoCbl riboswitches is thought to inhibit transcription termination as transcription terminator helices have been identified downstream of the riboswitch.[10] This suggests that unlike characterized AdoCbl riboswitches that usually inhibit cobalamin biosythesis by promoting transcription termination this newly identified AdoCbl riboswitch promotes the expression of downstream genes.
Other forms of cobalamin riboswitches bind aquocobalamin.[11]
Crystal structures of cobalamin riboswitches have been determined.[11][12]
References
- 1 2 3 Nahvi, A. S.; Sudarsan, N.; Ebert, M. S.; Zou, X.; Brown, K. L.; Breaker, R. R. (Sep 2002). "Genetic control by a metabolite binding mRNA". Chemistry & Biology. 9 (9): 1043–1049. doi:10.1016/S1074-5521(02)00224-7. ISSN 1074-5521. PMID 12323379.
- ↑ Franklund CV, Kadner RJ (June 1997). "Multiple transcribed elements control expression of the Escherichia coli btuB gene". J. Bacteriol. 179 (12): 4039–42. PMC 179215
 . PMID 9190822.
. PMID 9190822. - ↑ Vitreschak AG, Rodionov DA, Mironov AA, Gelfand MS (2003). "Regulation of the vitamin B12 metabolism and transport in bacteria by a conserved RNA structural element". RNA. 9 (9): 1084–97. doi:10.1261/rna.5710303. PMC 1370473
 . PMID 12923257.
. PMID 12923257. - ↑ Barrick JE, Breaker RR (2007). "The distributions, mechanisms, and structures of metabolite-binding riboswitches". Genome Biol. 8 (11): R239. doi:10.1186/gb-2007-8-11-r239. PMC 2258182
 . PMID 17997835.
. PMID 17997835. - ↑ Weinberg Z, Wang JX, Bogue J, et al. (March 2010). "Comparative genomics reveals 104 candidate structured RNAs from bacteria, archaea and their metagenomes". Genome Biol. 11 (3): R31. doi:10.1186/gb-2010-11-3-r31. PMC 2864571
 . PMID 20230605.
. PMID 20230605. - 1 2 3 Fox, A.; Ramesh, A.; Stearns, E.; Bourgogne, A.; Reyes-Jara, A.; Winkler, C.; Garsin, A. (Mar 2009). "Multiple posttranscriptional regulatory mechanisms partner to control ethanolamine utilization in Enterococcus faecalis". Proceedings of the National Academy of Sciences of the United States of America. 106 (11): 4435–4440. Bibcode:2009PNAS..106.4435F. doi:10.1073/pnas.0812194106. ISSN 0027-8424. PMC 2647976
 . PMID 19246383.
. PMID 19246383. - ↑ Randle, C. A.; Albro, P. W.; Dittmer, J. C. (1969). "The phosphoglyceride composition of Gram-negative bacteria and the changes in composition during growth" (Free full text). Biochimica et Biophysica Acta. 187 (2): 214–220. doi:10.1016/0005-2760(69)90030-7. ISSN 0006-3002. PMID 4898381.
- ↑ Del Papa, F.; Perego, M. (Nov 2008). "Ethanolamine Activates a Sensor Histidine Kinase Regulating Its Utilization in Enterococcus faecalis" (Free full text). Journal of Bacteriology. 190 (21): 7147–7156. doi:10.1128/JB.00952-08. ISSN 0021-9193. PMC 2580688
 . PMID 18776017.
. PMID 18776017. - ↑ Abreu-Goodger, C.; Merino, E. (Jul 2005). "RibEx: a web server for locating riboswitches and other conserved bacterial regulatory elements" (Free full text). Nucleic Acids Research. 33 (Web Server issue): W690–W692. doi:10.1093/nar/gki445. ISSN 0305-1048. PMC 1160206
 . PMID 15980564.
. PMID 15980564. - ↑ Nahvi, A.; Barrick, E.; Breaker, R. (2004). "Coenzyme B12 riboswitches are widespread genetic control elements in prokaryotes" (Free full text). Nucleic Acids Research. 32 (1): 143–150. doi:10.1093/nar/gkh167. ISSN 0305-1048. PMC 373277
 . PMID 14704351.
. PMID 14704351. - 1 2 Johnson JE, Reyes FE, Polaski JT, Batey RT (2012). "B12 cofactors directly stabilize an mRNA regulatory switch". Nature. 492 (7427): 133–7. doi:10.1038/nature11607. PMC 3518761
 . PMID 23064232.
. PMID 23064232. - ↑ Peselis A, Serganov A (2012). "Structural insights into ligand binding and gene expression control by an adenosylcobalamin riboswitch". Nat. Struct. Mol. Biol. 19 (11): 1182–4. doi:10.1038/nsmb.2405. PMID 23064646.