Covalent organic framework
The design and synthesis of crystalline extended organic structures in which the building blocks are linked by strong covalent bonds are core concepts of covalent organic frameworks (COFs). COFs are porous, and crystalline, and made entirely from light elements (H, B, C, N, and O) that are known to form strong covalent bonds in well-established and useful materials such as diamond, graphite, and boron nitride.
Preparation of COF materials from molecular building blocks would provide covalent frameworks that could be functionalized into lightweight materials for diverse applications.[1]
Structure
Porous crystalline solids consists of secondary building units (SBUs) which assemble to form a periodic and porous framework.
An almost infinite numbers of frameworks can be formed through various SBU combinations leading to unique material properties for applications in separations, storage, and heterogeneous catalysis.[2]
Porous crystalline solids can be used to describe materials such as Zeolite, Metal-organic frameworks (MOFs), and Covalent Organic Frameworks (COFs).
Zeolites are microporous, aluminosilicate minerals commonly used as commercial adsorbents.
MOFs are a class of porous polymeric material, consisting of metal ions linked together by organic bridging ligands and are a new development on the interface between molecular coordination chemistry and materials science.[3]
COFs are another class of porous polymeric materials, consisting of porous, crystalline, covalent bonds that usually have rigid structures, exceptional thermal stabilities (to temperatures up to 600 °C), and low densities. They exhibit permanent porosity with specific surface areas surpassing those of well-known zeolites and porous silicates.[1]
Secondary building units
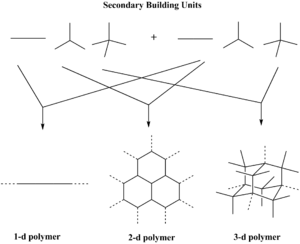
The term ‘secondary building unit’ has been used for some time to describe conceptual fragments which can be compared as bricks used to build a house of zeolites; in the context of this page it refers to the geometry of the units defined by the points of extension.[4] Recently, 279 of new secondary building units have been found on the crystal structure database.[5][6]
Reticular synthesis
Although the synthesis of new materials has long been recognized as the most essential element in advancing technology, it generally remains more of an art than a science — in that the discovery of new compounds has mostly been serendipitous, using methods referred to by critics as ‘shake and bake’, ‘mix and wait’, 'mash and smash' and ‘heat and beat’.[4] The reason is that the starting entities do not maintain their structure during the reaction, leading to poor correlation between reactants and products. However, the design of an extended network that will maintain its structural integrity throughout the construction process can be realized by starting with well-defined and rigid molecular building blocks.
In essence, reticular synthesis can be described as the process of assembling judiciously designed rigid secondary building units into predetermined ordered structures (networks), which are held together by strong bonding. It is different from retrosynthesis of organic compounds, because the structural integrity and rigidity of the building blocks in reticular synthesis remain unaltered throughout the construction process — an important aspect that could help to fully realize the benefits of design in crystalline solid-state frameworks. Similarly, reticular synthesis should be distinguished from supramolecular assembly, because in the former, building blocks are linked by strong bonds throughout the crystal.[4]
Applications
Hydrogen storage
Omar M. Yaghi and William A. Goddard III reported COFs as exceptional hydrogen storage materials. They predicted the highest excess H2 uptakes at 77 K are 10.0 wt % at 80 bar for COF-105, and 10.0 wt % at 100 bar for COF-108, which have higher surface area and free volume, by grand canonical Monte Carlo (GCMC) simulations as a function of temperature and pressure. This is the highest value reported for associative H2 storage of any material. Thus 3-D COFs are most promising new candidates in the quest for practical H2 storage materials.[7] In 2012, the lab of William A. Goddard III reported the uptake for COF102, COF103, and COF202 at 298 K and they also proposed new strategies to obtain higher interaction with H2. Such strategy consist on metalating the COF with alkaline metals such as Li.[8] These complexes composed of Li, Na and K with benzene ligands (such as 1,3,5-benzenetribenzoate, the ligand used in MOF-177) have been synthesized by Krieck et al.[9] and Goddard showed that the THF is important of their stability. If the metalation with alkaline is performed in the COFs, Goddard et al. calculated that some COFs can reach 2010 DOE gravimetric target in delivery units at 298 K of 4.5 wt %: COF102-Li (5.16 wt %), COF103-Li (4.75 wt %), COF102-Na (4.75 wt %) and COF103-Na (4.72 wt %). COFs also perform better in delivery units than MOFs because the best volumetric performance is for COF102-Na (24.9), COF102-Li (23.8), COF103-Na (22.8), and COF103-Li (21.7), all using delivery g H2/L units for 1–100 bar. These are the highest gravimetric molecular hydrogen uptakes for a porous material under these thermodynamic conditions.[8] Other strategies to increase the interaction of COFs with molecular hydrogen have been reviewed recently.[10] In 2012, the new COF-301-PdCl2 is predicted to reach 60 g total H2 /L at 100 bar, which is 1.5 times the DOE 2015 target of 40 g/L and close to the ultimate (2050) target of 70 g/L.[11]
Methane storage
Omar M. Yaghi and William A. Goddard III also reported COFs as exceptional methane storage materials. The best COF in terms of total volume of CH4 per unit volume COF absorbent is COF-1, which can store 195 v/v at 298 K and 30 bar, exceeding the U.S. Department of Energy target for CH4 storage of 180 v/v at 298 K and 35 bar. The best COFs on a delivery amount basis (volume adsorbed from 5 to 100 bar) are COF-102 and COF-103 with values of 230 and 234 v(STP: 298 K, 1.01 bar)/v, respectively, making these promising materials for practical methane storage.[12] More recently, new COFs with better delivery amount have been designed in the lab of William A. Goddard III, and they have been shown to be stable and overcome the DOE target in delivery basis. COF-103-Eth-trans and COF-102-Ant, are found to exceed the DOE target of 180 v(STP)/v at 35 bar for methane storage. They reported that using thin vinyl bridging groups aid performance by minimizing the interaction methane-COF at low pressure. This is a new feature that can be used to enhance loading in addition to the common practice of adding extra fused benzene rings.[13]
Optical properties
A highly ordered π-conjugation TP-COF, consisting of pyrene and triphenylene functionalities alternately linked in a mesoporous hexagonal skeleton, is highly luminescent, harvests a wide wavelength range of photons, and allows energy transfer and migration. Furthermore, TP-COF is electrically conductive and capable of repetitive on–off current switching at room temperature.[14]
Porosity/surface-area effects
Most studies to date have focused on the development of synthetic methodologies with the aim of maximizing pore size and surface area for gas storage. That means the functions of COFs have not yet been well explored, but COFs can be used as catalyst, or gas separation etc.[1]
Carbon capture
In 2015 the use of highly porous, catalyst-decorated COFs for converting carbon dioxide into carbon monoxide.[15]
History
While at UMich, Omar M. Yaghi (currently at UCBerkeley) and Adrien P Cote published the first paper of COF.[1] They reported the design and successful synthesis of COFs by condensation reactions of phenyl diboronic acid {C6H4[B(OH)2]2} and hexahydroxytriphenylene [C18H6(OH)6]. Powder X-ray diffraction studies of the highly crystalline products (C3H2BO)6&(C9H12)1 (COF-1) and C9H4BO2 (COF-5) revealed 2-dimensional expanded porous graphitic layers that are either staggered (COF-1, P63/mmc) or eclipsed (COF-5, P6/mmm). Their crystal structures are entirely held by strong bonds between B, C, and O atoms to form rigid porous architectures with pore sizes ranging from 7 to 27 angstroms. COF-1 and COF-5 exhibit high thermal stability (to temperatures up to 500 to 600 C), permanent porosity, and high surface areas (711 and 1590 square meters per gram, respectively).[1]
The synthesis of 3D COFs has been hindered by longstanding practical and conceptual challenges. Unlike 0D and 1D system, the insolubility of 2D and 3D structures precludes the use of stepwise synthesis, making their isolation in crystalline form very difficult. The first challenge, however, was overcome by judiciously choosing building blocks and using reversible condensation reactions to crystallize COFs. Examples of 3D COFs are COF-102, 103, 105, 108, 202, and 300. Most of 3D COF show high surface area, which surpass those of 2D (3472, 4210, 3214, square meters per gram for COF-102, 103, and 202 respectively). COF-105 and 108 calculated theoretically to perform exceptional hydrogen storage function which is the highest value reported for associative H2 storage of any material.[16]
Synthetic Chemistry of COFs
Boron condensation
The most popular COF synthesis route is a boron condensation reaction which is a molecular dehydration reaction between boronic acids. In case of COF-1, three boronic acid molecules converge to form a planar six-membered B3O3 (boroxine) ring with the elimination of three water molecules.[1]

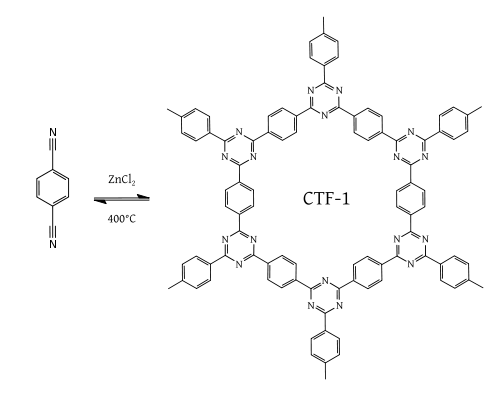
Triazine based trimerization
Another class of high performance polymer frameworks with regular porosity and high surface area is based on triazine materials which can be achieved by dynamic trimerization reaction of simple, cheap, and abundant aromatic nitriles in ionothermal conditions (molten zinc chloride at high temperature (400 °C)). CTF-1 is a good example of this chemistry.[17]
Imine condensation
A new class of COFs can be obtained by imine condensation of aniline with benzaldehyde that results in imine bond formation with elimination of water. COF-300 is a good example of this chemistry.[18]
Characterization
Many COFs lack long-range order, meaning they are much harder to characterise through the use of diffraction techniques. However, powder X-ray diffraction (PXRD) is very useful for crystalline materials, allowing the determination of their crystal structures. However, the relatively long wavelength used in conventional laboratory sources means they are unable to properly characterise the short range order of amorphous/non-crystalline COFs. Morphology can be probed through scanning electron microscopy (SEM). Finally, porosity, in most cases surface area, is measured by a N2 sorption isotherm.
See also
References
- 1 2 3 4 5 6 Côté, A. P.; Benin, A. I.; Ockwig, N. W.; O'Keeffe, M.; Matzger, A. J.; Yaghi, O. M.; Porous, Crystalline, Covalent Organic Frameworks. Science. 2005, 310, pp 1166-1170. doi:10.1126/science.1120411
- ↑ Kitagawa, S.; Kitaura, R.; Noro, S.; Functional Porous Coordination Polymers. Angew. Chem. Int. Ed. 2004, 43, pp 2334-2375. doi:10.1002/anie.200300610
- ↑ James, S. L.; Metal-organic frameworks. Chem. Soc. Rev. 2003, 32, pp 276-288. doi:10.1039/B200393G
- 1 2 3 Yaghi, O. M.; O'Keeffe, M.; Ockwig, N. W.; Chae, H. K.; Eddaoudi, M.; Kim, J.; Reticular synthesis and the design of new materials. Nature. 2003, 423, pp 705-714. doi:10.1038/nature01650
- ↑ Tranchemontagne David J.; Mendoza-Cortes Jose L.; O'Keeffe Michael; Yaghi OM; Secondary building units, nets and bonding in the chemistry of metal-organic frameworks. Chemical Society Reviews. 2009, 38, pp 1257-1283. doi:10.1039/b817735j
- ↑ Tranchemontagne David J.; Mendoza-Cortes Jose L.; O'Keeffe Michael; Yaghi OM; Secondary building units, nets and bonding in the chemistry of metal-organic frameworks . Supplementary material
- ↑ Han, S.; Hurukawa, H.; Yaghi, O. M.; Goddard, W. A.; Covalent Organic Frameworks as Exceptional Hydrogen Storage Materials. J. Am. Chem. Soc. 2008, 130, pp 11580–11581. doi:10.1021/ja803247y
- 1 2 Mendoza-Cortes, JL; Han, SS; Goddard, WA; High H2 Uptake in Li-, Na-, K-Metalated Covalent Organic Frameworks and Metal Organic Frameworks at 298 K. J. Phys. Chem. A. 2012, 116, pp 1621–1631. doi:10.1021/jp206981d
- ↑ Krieck, S.; Gorls, H.; Westerhausen, M., Alkali Metal-Stabilized 1,3,5-Triphenylbenzene Monoanions: Synthesis and Characterization of the Lithium, Sodium, and Potassium Complexes. Organometallics. 2010, 29, pp 6790–6800. doi:10.1021/om1009632
- ↑ Han SS; Mendoza-Cortes JL; Goddard WA.; Recent advances on simulation and theory of hydrogen storage in metal–organic frameworks and covalent organic frameworks. Chemical Society Reviews. 2009, 38, pp 1460-1476. doi:10.1039/B802430H
- ↑ Mendoza-Cortes, JL; Goddard, WA; Furukawa, H.; Yaghi OM.; A Covalent Organic Framework that Exceeds the DOE 2015 Volumetric Target for H2 Uptake at 298 K. Journal of Physical Chemistry Letters. 2012, 18, pp 2671–2675. doi:10.1021/jz301000m
- ↑ Mendoza-Cortes JL; Han SS; Furukawa H; Yaghi OM; Goddard, WA; Adsorption Mechanism and Uptake of Methane in Covalent Organic Frameworks: Theory and Experiment. J. Phys. Chem. A, 2010, 114, pp 10824–10833. doi:10.1021/jp1044139
- ↑ Mendoza-Cortes, JL; Pascal, TA; Goddard, WA; Design of Covalent Organic Frameworks for Methane Storage. J. Phys. Chem. A, 2011, 115, pp 13852–13857. doi:10.1021/jp209541e
- ↑ Shun, W.; Jia, G.; Jangbae, K.; Hyotcherl, I.; Donglin, J.; A Belt-Shaped, Blue Luminescent, and Semiconducting Covalent Organic Framework. Angew. Chem. Int. Ed. 2008, 47, pp 8826-8830. doi:10.1002/anie.200890235
- ↑ Martin, Richard (September 24, 2015). "New Technology to Capture, Convert Carbon Dioxide | MIT Technology Review". Retrieved 2015-09-27.
- ↑ El-Kaderi, H. M.; Hunt, J. R.; Mendoza-Cortés, J.; Côté, A.; Taylor, R. E.; O'Keeffe, M.; Yaghi1, O. M.; Designed Synthesis of 3D Covalent Organic Frameworks. Science. 2007, 316, pp 268-272. doi:10.1126/science.1139915
- ↑ Kuhn, P.; Antonietti, M.; Thomas, A.; Porous, Covalent Triazine-Based Frameworks Prepared by Ionothermal Synthesis. Angew. Chem. Int. Ed. 2008. 47, pp 3450-3453. PMID 18330878
- ↑ Uribe-Romo, F. J.; Hunt, J. R.; Furukawa, H.; Klck, C.; O’Keeffe, M.; Yaghi, O. M.; A Crystalline Imine-Linked 3-D Porous Covalent Organic Framework. J. Am. Chem. Soc. 2009, 131, pp 4570-4571. doi:10.1021/ja8096256