Cyanoacrylate
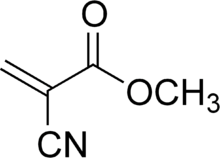
Cyanoacrylates are a family of strong fast-acting adhesives with industrial, medical, and household uses. Cyanoacrylate adhesives have a short shelf life if not used, about one year from manufacture if unopened, one month once opened. They have some minor toxicity.
Cyanoacrylates include methyl 2-cyanoacrylate, ethyl-2-cyanoacrylate (commonly sold under trade names such as "Super Glue" and "Krazy Glue"), n-butyl cyanoacrylate and 2-octyl cyanoacrylate (used in medical, veterinary and first aid applications). Octyl cyanoacrylate was developed to address toxicity concerns and to reduce skin irritation and allergic response. Cyanoacrylate adhesives are sometimes known generically as instant glues, power glues or superglues (although "Super Glue" is a trade name).[1] The abbreviation "CA" is commonly used for industrial grades.
Development

The original patent for cyanoacrylate was filed in 1942 by Goodrich Company.[2] as an outgrowth of a search for materials suitable for clear plastic gun sights for the war effort. In 1942, a team of scientists headed by Harry Coover Jr. stumbled upon a formulation that stuck to everything with which it came in contact.[3] The team quickly rejected the substance for the wartime application, but in 1951, while working as researchers for Eastman Kodak, Coover and a colleague, Fred Joyner, rediscovered cyanoacrylates. The two realized the true commercial potential, and a form of the adhesive was first sold in 1958 under the title "Eastman #910" (later "Eastman 910").
During the 1960s, Eastman Kodak sold cyanoacrylate to Loctite, which in turn repackaged and distributed it under a different brand name "Loctite Quick Set 404". In 1971 Loctite developed its own manufacturing technology and introduced its own line of cyanoacrylate, called "Super Bonder". Loctite quickly gained market share, and by the late 1970s it was believed to have exceeded Eastman Kodak's share in the North American industrial cyanoacrylate market. National Starch and Chemical Company purchased Eastman Kodak’s cyanoacrylate business and combined it with several acquisitions made throughout the 1970s forming Permabond. Other manufacturers of cyanoacrylate include LePage (a Canadian company acquired by Henkel in 1996), the Permabond Division of National Starch and Chemical, Inc., which was a subsidiary of Unilever. Together, Loctite, Eastman and Permabond accounted for approximately 75% of the industrial cyanoacrylate market.[4] As of 2013 Permabond continued to manufacture the original 910 formula.
Properties
In its liquid form, cyanoacrylate consists of monomers of cyanoacrylate molecules. Methyl-2-cyanoacrylate (CH2=C(CN)COOCH3 or C5H5NO2) has a molecular weight equal to 111.1, a flashpoint of 79 °C, and a density of 1.1 g/ml.[5] Ethyl 2-cyanoacrylate (C6H7NO2) has a molecular weight equal to 125 and a flashpoint of >75 °C. To facilitate easy handling, a cyanoacrylate adhesive is frequently formulated with an ingredient such as fumed silica to make it more viscous or gel-like. More recently, formulations are available with additives to increase shear strength, creating a more impact resistant bond. Such additives may include rubber, as in Loctite's Ultra Gel, or others which are not specified.
In general, cyanoacrylate is an acrylic resin that rapidly polymerises in the presence of water (specifically hydroxide ions), forming long, strong chains, joining the bonded surfaces together. Because the presence of moisture causes the glue to set, exposure to normal levels of humidity in the air causes a thin skin to start to form within seconds, which very greatly slows the reaction. Because of this cyanoacrylate is applied thinly, to ensure that the reaction proceeds rapidly for bonding.
The reaction with moisture can cause a container of glue which has been opened and resealed to become unusable more quickly than if never opened. To minimise this reduction in shelf life cyanoacrylate, once opened, can be stored in an airtight container with a package of silica gel desiccant. Another technique is to insert a hypodermic needle into the opening of a tube. After using the glue, residual glue soon clogs the needle, keeping moisture out. The clog is removed by heating the needle (e.g. with a lighter) before use.. The polymerisation is also temperature-dependant: storage at zero degrees or below stops it, so keeping it in the freezer is also effective.

Uses

Behaviours
Cyanoacrylates are mainly used as adhesives. They require some care and knowledge for effective use: they do not bond some materials; their shelf life at room temperature is about 12 months unopened and one month once opened; they do not fill spaces, unlike epoxies, and a very thin layer bonds more effectively than a thicker one that does not cure properly; they bond many substances, including human skin and tissues. They have an exothermic reaction to natural fibres: cotton, wool, leather, see reaction with cotton below.
Cyanoacrylate glue has a low shearing strength, which has led to its use as a temporary adhesive in cases where the piece needs to be sheared off later. Common examples include mounting a workpiece to a sacrificial glue block on a lathe, and tightening pins and bolts. It is also used in conjunction with another, slower, but more resilient adhesive as way of rapidly forming a joint, which then holds the pieces in the appropriate configuration until the second adhesive has set.
Cyanoacrylate is not resistant to friction if applied to a smooth surface. Using either sugar or sandpaper can remove a good amount of cyanoacrylate from a user's fingertips.
Electronics
Cyanoacrylates are used to assemble prototype electronics (see wire wrap), flying model aircraft, and as retention dressings for nuts and bolts. Their effectiveness in bonding metal and general versatility have made them popular among modeling and miniatures hobbyists.
Aquaria
Cyanoacrylate glue's ability to resist water has made it popular with marine aquarium hobbyists for fragging corals. The cut branches of hard corals such as Acropora can be glued to a piece of live rock (harvested reef coral) or Milliput (epoxy putty) to allow the new frag to grow out. It is safe to use directly in the tank, unlike silicone, which must be cured to be safe. However, as a class of adhesives, traditional cyanoacrylates are classified as having "weak" resistance to both moisture and heat[6] although the inclusion of phthalic anhydride reportedly counteracts both of these characteristics.[7]
Bonding smooth surfaces
Most standard cyanoacrylate adhesives do not bond well with smooth glass, although they can be used as a quick, temporary bond prior to application of an epoxy or cyanoacrylate specifically formulated for use on glass.[8] A mechanical adhesive bond may be formed around glass fibre mat or tissue to reinforce joints or to fabricate small parts.
Filler
When added to baking soda (sodium bicarbonate), cyanoacrylate glue forms a hard, lightweight adhesive filler (baking soda is first used to fill a gap then the adhesive is dropped onto the baking soda). This works well with porous materials that the glue does not work well with alone. This method is sometimes used by aircraft modelers to assemble or repair polystyrene foam parts. It is also used to repair small nicks in the leading edge of composite propeller blades on light aircraft. The reaction between cyanoacrylate and baking soda is very exothermic (heat-producing) and also produces noxious vapors. See Reaction with cotton and wool below.
Forensics
Cyanoacrylate is used as a forensic tool to capture latent fingerprints on non-porous surfaces like glass, plastic, etc.[9] Cyanoacrylate is warmed to produce fumes that react with the invisible fingerprint residues and atmospheric moisture to form a white polymer (polycyanoacrylate) on the fingerprint ridges. The ridges can then be recorded. The developed fingerprints are, on most surfaces (except on white plastic or similar), visible to the naked eye. Invisible or poorly visible prints can be further enhanced by applying a luminescent or non-luminescent stain.
Woodworking
Thin CA glue has application in woodworking. It can be used as a fast-drying, glossy finish. The use of oil (such as boiled linseed oil) may be used to control the rate at which the CA cures. CA glue is also used in combination with sawdust (from a saw or sanding) to fill voids and cracks. These repair methods are used on piano soundboards, wood instruments, and wood furniture.
Medical
CA glue was in veterinary use for mending bone, hide, and tortoise shell by the early 1970s or before. Harry Coover said in 1966 that a CA spray was used in the Vietnam War to reduce bleeding in wounded soldiers until they could be brought to a hospital. n-Butyl cyanoacrylate has been used medically since the 1970s. In the US, due to its potential to irritate the skin, the U.S. Food and Drug Administration did not approve its use as a medical adhesive until 1998 with Dermabond.[10] Studies confirm that cyanoacrylate is safer and more functional for wound closure than traditional suturing (stitches).[11] The adhesive is superior in time required to close wounds, incidence of infection (suture canals through the skin's epidermal, dermal, and subcutaneous fat layers introduce extra routes of contamination),[11] and final cosmetic appearance.[12][13]
Some rock climbers use cyanoacrylate to repair damage to the skin on their fingertips.[14][15] Similarly, stringed-instrument players can form protective finger caps (in addition to calluses) with cyanoacrylates. While the glue is not very toxic and wears off quickly with shed skin, applying large quantities of glue and its fumes directly to the skin can cause chemical burns.
While standard "superglue" is 100% ethyl cyanoacrylate, many custom formulations (e.g., 91% ECA, 9% poly(methyl methacrylate), <0.5% hydroquinone, and a small amount of organic sulfonic acid,[16] and variations on the compound n-butyl-cyanoacrylate for medical applications[11]) have come to be used for specific applications. There are 3 cyanoacrylate compounds currently available as topical skin adhesives. 2-Octyl-cyanoacrylate is marketed as Dermabond and SurgiSeal. n-2-Butyl-cyanoacrylate is marketed as Histoacryl, Indermil, GluStitch, GluSeal, PeriAcryl, and LiquiBand. The compound 2-Ethyl-cyanoacrylate is available as Epiglu.[17]
Archery
Cyanoacrylate is used in archery to glue fletching to arrow shafts. Some special fletching glues are primarily cyanoacrylate repackaged in special fletching glue kits. Such tubes often have a long thin metal nozzle for improved precision in applying the glue to the base of the fletching and to ensure secure bonding to the arrow shaft.
Cosmetics
Cyanoacrylate is used in the cosmetology/beauty industry as a "nail glue" for some artificial nail enhancements such as nail tips and nail wraps, and is sometimes mistaken for eye drops causing accidental injury.[18]
Safety issues
Skin injuries
CA adhesives may adhere to body parts, and injuries may occur when parts of the skin are torn off.[19][20] Without force, however, the glue will spontaneously separate from the skin in time (up to four days). Separation can be accelerated by applying vegetable oil near, on, and around the glue. In the case of glued eyelids, a doctor should be consulted.[21]
Toxicity
The fumes from CA are a vaporized form of the cyanoacrylate monomer that irritate sensitive membranes in the eyes, nose, and throat. They are immediately polymerized by the moisture in the membranes and become inert. These risks can be minimized by using CA in well ventilated areas. About 5% of the population can become sensitized to CA fumes after repeated exposure, resulting in flu-like symptoms.[22] CA may also be a skin irritant, causing an allergic skin reaction. The ACGIH assign a Threshold Limit Value exposure limit of 200 parts per billion. On rare occasions, inhalation may trigger asthma. There is no singular measurement of toxicity for all cyanoacrylate adhesives because of the large number of adhesives that contain various cyanoacrylate formulations.
The United Kingdom's Health and Safety Executive and the United States National Toxicology Program have concluded that the use of ethyl cyanoacrylate is safe and that additional study is unnecessary.[23] The compound 2-octyl cyanoacrylate degrades much more slowly due to its longer organic backbone and the adhesive does not reach the threshold of tissue toxicity. Due to the toxicity issues of ethyl cyanoacrylate, the use of 2-octyl cyanoacrylate for sutures is preferred.
Reaction with cotton and wool
Applying cyanoacrylate to some natural materials such as cotton, leather or wool (cotton swabs, cotton balls, and certain yarns or fabrics) results in a powerful, rapid exothermic reaction. The heat released may cause serious burns,[24] ignite the cotton product, or release irritating white smoke. Material Safety Data Sheets for cyanoacrylate instruct users not to wear cotton or wool clothing, especially cotton gloves, when applying or handling cyanoacrylates.[25]
Solvents and debonders
Acetone, commonly found in nail polish remover, is a widely available solvent capable of softening cured cyanoacrylate.[26] Other solvents include nitromethane, dimethyl sulfoxide, and methylene chloride.[27] gamma-Butyrolactone may also be used to remove cured cyanoacrylate.[28] Commercial debonders are also available.[29]
Shelf life
CA adhesives have a short shelf life. Date-stamped containers help to ensure that the adhesive is still viable. One manufacturer supplies the following information and advice:
When kept unopened in a cool, dry location such as a refrigerator at a temperature of about 55 °F (13 °C), the shelf life of cyanoacrylate will be extended from about one year from manufacture to at least 15 months. If the adhesive is to be used within six months, it is not necessary to refrigerate it. Cyanoacrylates are moisture-sensitive, and moving from a cool to a hot location will create condensation; after removing from the refrigerator, it is best to let the adhesive reach room temperature before opening. After opening, it should be used within 30 days. Open containers should not be refrigerated.[30]
Another manufacturer says that the maximum shelf life of 12 months is obtained for some of their cyanoacrylates if the original containers are stored at 35 to 40 °F (2 to 4 °C).[31] User forums and some manufacturers say that an almost unlimited shelf life is attainable by storing unopened at −4 °F (−20 °C), the typical temperature of a domestic freezer, and allowing the contents to reach room temperature before use.[32] Rechilling an opened container may cause moisture from the air to condense in the container; however, reports from hobbyists suggest that storing CA in a freezer can preserve opened cyanoacrylate indefinitely.
As cyanoacrylates age, they polymerize, become thicker, and cure more slowly. They can be thinned with a cyanoacrylate of the same chemical composition with lower viscosity.[33] Storing cyanoacrylates below 0 °F (−18 °C) will nearly stop the polymerization process and prevent aging.
See also
- Methyl cyanoacrylate
- Ethyl cyanoacrylate
- Butyl cyanoacrylate
- Octyl cyanoacrylate
- 2-Octyl cyanoacrylate
References
- ↑ Oxford English Dictionary, 2nd ed. cites "Croid Super Glue can be used..." (1937); "‘Gunk’ is what workers in the Chrysler Corporation factory call their superglue named Cycleweld" (1944)
- ↑ Ardis, Alan. "Patent for synthesis of cyanoacrylate". Google patents. Google. Retrieved 22 February 2016.
- ↑ "Inventor of the Week Archive". Lemelson-MIT Program. September 2004. Archived from the original on 3 May 2009. Retrieved 13 February 2010.
- ↑ HBS, "Loctite Corporation: Industrial Product Group," 15 July 1991, p.3
- ↑ "ICSC 1272 - METHYL 2-CYANOACRYLATE".
- ↑ Petrie, Edward M. (2000). Handbook of adhesives and sealants. New York: McGraw-Hill. p. 354. ISBN 0-07-049888-1.
- ↑ Petrie, Edward M. (2000). Handbook of adhesives and sealants. New York: McGraw-Hill. p. 389. ISBN 0-07-049888-1.
- ↑ "Archived copy". Archived from the original on 3 December 2014. Retrieved 11 August 2010.
- ↑ Eric W. Brown "The Cyanoacrylate Fuming Method"
- ↑ Singer, A. J.; McClain, S. A.; Katz, A. (2004). "A porcine epistaxis model: hemostatic effects of octylcyanoacrylate". Otolaryngology-Head and Neck Surgery. 130 (5): 553–557. doi:10.1016/j.otohns.2003.09.035. PMID 15138419.
- 1 2 3 Dalvi, A A; Faria, M M; Pinto, A A (1986). "Non-suture closure of wound using cyanoacrylate". J Postgrad Med [serial online]. 32: 97–100.
- ↑ Fischl, Robert A. (November 1962). "An adhesive for primary closure of skin incisions: a preliminary report". Plastic and Reconstructive Surgery. 30 (6): 607–610. doi:10.1097/00006534-196211000-00009. PMID 13963055.
- ↑ Rothnie, N. G.; Taylor, G. W. (26 October 1963). "Sutureless Skin Closure". BMJ. 2 (5364): 1027–1030. doi:10.1136/bmj.2.5364.1027. PMC 1873305
 . PMID 14056909.
. PMID 14056909. - ↑ "Bouldering". climbingaction.com. Retrieved 19 February 2011.
- ↑ Anahad O'Connor (4 December 2007). "The Claim: Super Glue Can Heal Wounds". The New York Times. Retrieved 19 February 2011.
- ↑ Safety data for ethyl cyanoacrylate from the Physical and Theoretical Chemistry Laboratory of Oxford University
- ↑ "Topical skin adhesives. DermNet NZ". www.dermnetnz.org. Retrieved 2016-06-29.
- ↑ Needham, A. D (2001). "Similarities in the packaging of cyanoacrylate nail glue and ophthalmic preparations: an ongoing problem". British Journal of Ophthalmology. 85 (4): 496a–496. doi:10.1136/bjo.85.4.496a. ISSN 0007-1161.
- ↑ Clarke, T.F.E. (2011). "Cyanoacrylate glue burn in a child – lessons to be learned". Journal of Plastic, Reconstructive & Aesthetic Surgery. 64 (7): e170–e173. doi:10.1016/j.bjps.2011.03.009. ISSN 1748-6815. PMID 21481658.
- ↑ . doi:10.3980/j.issn.2222-3959.2012.05.18. Missing or empty
|title=(help) - ↑ First aid information (in German) by Industrieverband Klebstoffe e.V.
- ↑ "CA PLUS Adhesives, Inc.".
- ↑ Methyl Cyanoacrylate and Ethyl Cyanoacrylate from inchem.org
- ↑ Clarke, TFE (March 2011). "Superglue (Cyanoacrylate) in the Nose". Journal of Plastic, Reconstructive & Aesthetic Surgery. 64 (7): e170–3. doi:10.1016/j.bjps.2011.03.009. PMID 21481658.
- ↑ "Material Safety Data Sheet" (PDF). accumetricinc.com. Archived from the original (PDF) on 19 February 2009. Retrieved 9 June 2008.
- ↑ Moschos, M.; Droutsas, D.; Boussalis, P.; Tsioulias, G. (1997). "Clinical experience with cyanoacrylate tissue adhesive". Documenta Ophthalmologica. Soringer. 93 (3): 237–245. doi:10.1007/BF02569064. PMID 9550352. Retrieved 29 March 2011.
- ↑ Duvvi, Sham K.; Lo, Stephen; Kumar, R; Spraggs, P (2005). "Superglue (Cyanoacrylate) in the Nose". Otolaryngology-Head and Neck Surgery. 133 (5): 803–804. doi:10.1016/j.otohns.2004.09.090. Retrieved 29 March 2011.
- ↑ Shantha, K.L.; Krishnamurti, N.; Krishnamurti, N. (1989). "Developments and applications of cyanoacrylate adhesives". Journal of Adhesion Science and Technology. VSP. 3 (1): 237–260. doi:10.1163/156856189X00191. Retrieved 29 March 2011.
- ↑ "Product Review". nail designary review.
- ↑ Palm Labs Adhesives: Cyanoacrylate Adhesive Shelf Life
- ↑ MASTER BOND MB SERIES CYANOACRYLATES: Technical Data Sheet
- ↑ WEICON Contact Cyanoacrylate Adhesives
- ↑ "CA PLUS Adhesives, Inc.".
Further reading
- derma+flex QS 510k Letter: http://www.accessdata.fda.gov/cdrh_docs/pdf10/K101276.pdf
- LiquiBand 510k Letter: http://www.accessdata.fda.gov/cdrh_docs/pdf8/K083531.pdf
- Fernandez, Tania & Bliskovsky, Val (2 January 2003). "Cyanoacrylate Technology: Stay Glued". Pharmbiz.com.
- Hayes, Sharon Caskey (11 July 2004). "Discovery of Super Glue helped land Coover in National Inventors Hall of Fame". Kingsport Times-News.
- Jueneman, F. (August 1981). "Stick it to um". Industrial Research & Development. p. 19.
- Perry, L. C. "An evaluation of acute incisional strength with Traumaseal surgical tissue adhesive wound closure". Dimensional Analysis Systems Inc.
- Quinn, J. & Kissack, J. (1994). "Tissue Adhesives for Laceration Repair During Sporting Events". Clinical Journal of Sports Medicine. 4 (4): 245–248. doi:10.1097/00042752-199410000-00006.
- Schwade, Nathan D. (10 April 2002). "Wound Adhesives, 2-Octyl Cyanoacrylate". eMedicine article.
- Vinters, H. V.; Galil, K. A.; Lundie, M. J.; Kaufmann, J. C. (1985). "The histotoxicity of cyanoacrylates. A selective review". Neuroradiology. 27 (4): 279–291. doi:10.1007/BF00339559. PMID 3900798.
External links
- Was Super Glue invented to seal battle wounds in Vietnam? (from The Straight Dope)
- Cyanoacrylate Toxicity
- Cyanoacrylate Adhesive / Super Glue Safety Data Sheets
- Safety in the Home: Super Glue - Queensland Health
- Cyanoacrylate Technical Data Sheet
- U.S. Patent 2,768,109 Alcohol-Catalyzed α-Cyanoacrylate Adhesive Compositions, filed June 1954, issued October 1956.
- 3M Activators, Primers and Debonder
- Application note on measuring cure kinetics of cyanoacrylate glues