Demoxytocin
 | |
| Clinical data | |
|---|---|
| Routes of administration | Buccal |
| ATC code | H01BB01 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms |
ODA-914; 1-mercaptopropionate- oxytoxin |
| CAS Number |
113-78-0 |
| PubChem (CID) | 449224 |
| ChemSpider |
395815 |
| ECHA InfoCard | 100.003.668 |
| Chemical and physical data | |
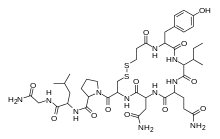
| Formula | C43H65N11O12S2 |
| Molar mass | 992.1727 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Demoxytocin (INN) (brand names Sandopart, Odeax, Sandopral), also known as desaminooxytocin or deaminooxytocin, as well as 1-(3-mercaptopropanoic acid)oxytocin ([Mpa1]OT), is an oxytocic peptide drug and synthetic analogue of oxytocin that is marketed in several European countries, including Italy, Czechoslovakia, and Poland.[1][2][3] It has the amino acid sequence Mpa-Tyr-Ile-Gln-Asn-Cys-Pro-Leu-Gly-NH2 (Mpa = β-mercaptopropionic acid),[4] and is an analogue of oxytocin wherein the leading cysteine is replaced with β-mercaptopropionic acid.[4] Demoxytocin has similar activities as oxytocin,[5] but is more potent and has a longer half-life in comparison.[3][4] Unlike oxytocin, which is given via intravenous injection, demoxytocin is administered as a buccal tablet formulation.[3][6] It is used to induce labor,[4] promote lactation,[3] and to prevent and treat puerperal (postpartum) mastitis (breast inflammation).[7] The drug was first synthesized in 1960 and was introduced into clinical practice in 1971 by Sandoz.[2][8]
See also
References
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 301–. ISBN 978-3-88763-075-1.
- 1 2 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 349–. ISBN 978-1-4757-2085-3.
- 1 2 3 4 I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 93–. ISBN 978-94-011-4439-1.
- 1 2 3 4 Christine Bladon (3 April 2002). Pharmaceutical Chemistry: Therapeutic Aspects of Biomacromolecules. John Wiley & Sons. pp. 61–. ISBN 978-0-471-49637-3.
- ↑ C.J. van Boxtel; B. Santoso; I.R. Edwards (6 August 2008). Drug Benefits and Risks: International Textbook of Clinical Pharmacology - Revised 2nd Edition. IOS Press. pp. 389–. ISBN 978-1-60750-345-3.
- ↑ Tom K. A. B. Eskes; Mieczyslaw Finster (22 October 2013). Drug Therapy During Pregnancy. Elsevier. pp. 185–. ISBN 978-1-4831-6298-0.
- ↑ Sternadel Z, Gerkowicz J (1979). "[Use of deaminooxytocin (Sandopart) in the prevention and treatment of puerperal mastitis]". Ginekol. Pol. (in Polish). 50 (5): 413–6. PMID 468056.
- ↑ Erhard Gross; Johannes Meienhofer (10 May 2014). Major Methods of Peptide Bond Formation: The Peptides Analysis, Synthesis, Biology. Elsevier. pp. 53–. ISBN 978-1-4832-1796-3.