Tolvaptan
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Samsca, Jinarc |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609033 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | C03XA01 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Unknown (40% absorbed) |
| Protein binding | 99% |
| Metabolism | Hepatic (CYP3A4-mediated)[1] |
| Biological half-life | 12 hours (terminal) |
| Identifiers | |
| |
| Synonyms | OPC-41061 |
| CAS Number |
150683-30-0 |
| PubChem (CID) | 216237 |
| IUPHAR/BPS | 2226 |
| ChemSpider |
187438 |
| UNII |
21G72T1950 |
| ChEMBL |
CHEMBL344159 |
| ECHA InfoCard | 100.219.212 |
| Chemical and physical data | |
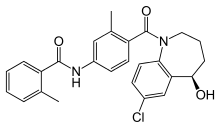
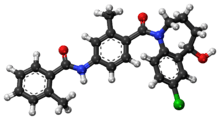
| Formula | C26H25ClN2O3 |
| Molar mass | 448.941 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Tolvaptan (INN, trade names Samsca and Jinarc) is a selective, competitive vasopressin receptor 2 antagonist used to treat hyponatremia (low blood sodium levels) associated with congestive heart failure, cirrhosis, and the syndrome of inappropriate antidiuretic hormone (SIADH). Tolvaptan was approved by the U.S. Food and Drug Administration (FDA) on May 19, 2009, and is sold by Otsuka Pharmaceutical Co. under the trade name Samsca and in India is manufactured & sold by MSN laboratories Ltd. under the trade name Tolsama & Tolvat and by Lupin under the brand name Resodim.
Tolvaptan was also in fast-track clinical trials[2] for polycystic kidney disease. In a 2004 trial, tolvaptan, when administered with traditional diuretics, was noted to increase excretion of excess fluids and improve blood sodium levels in patients with heart failure without producing side effects such as hypotension (low blood pressure) or hypokalemia (decreased blood levels of potassium) and without having an adverse effect on kidney function.[3] In a recently published trial (TEMPO 3:4 ClinicalTrials.gov number, NCT00428948) the study met its primary and secondary end points. Tolvaptan, when given at an average dose of 95 mg per day over a 3-year period, slowed the usual increase in kidney volume by 50% compared to placebo (2.80% per year versus 5.51% per year, respectively, p<0.001) and reduced the decline in kidney function when compared with that of placebo-treated patients by approximately 30% (reciprocal serum creatinine, -2.61 versus -3.81 (mg/mL)-1 per year, p <0.001)[4]
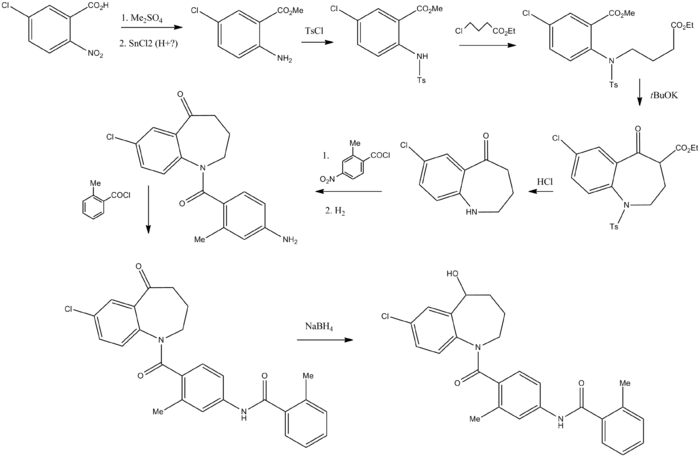
Synthesis
Side effects
FDA has determined that the drug Samsca (tolvaptan) should not be used for longer than 30 days and should not be used in patients with underlying liver disease because it can cause liver injury, potentially leading to liver failure[6]
References
- ↑ Shoaf S, Elizari M, Wang Z, et al. (2005). "Tolvaptan administration does not affect steady state amiodarone concentrations in patients with cardiac arrhythmias". J Cardiovasc Pharmacol Ther. 10 (3): 165–71. doi:10.1177/107424840501000304. PMID 16211205.
- ↑ Otsuka Maryland Research Institute, Inc.
- ↑ Gheorghiade M, Gattis W, O'Connor C, et al. (2004). "Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial". JAMA. 291 (16): 1963–71. doi:10.1001/jama.291.16.1963. PMID 15113814.
- ↑ (2012) Tolvaptan in Patients with Autosomal Dominant Polycystic Kidney Disease
- ↑ Kondo, K.; Ogawa, H.; Yamashita, H.; Miyamoto, H.; Tanaka, M.; Nakaya, K.; Kitano, K.; Yamamura, Y.; Nakamura, S.; Onogawa, T.; Mori, T.; Tominaga, M. (1999). "7-Chloro-5-hydroxy-1-[2-methyl-4-(2-methylbenzoylamino)benzoyl]-2,3,4,5-tetrahydro-1H-1-benzazepine (OPC-41061): A potent, orally active nonpeptide arginine vasopressin V2 receptor antagonist". Bioorganic & Medicinal Chemistry. 7 (8): 1743. doi:10.1016/S0968-0896(99)00101-7.
- ↑ "U.S. Food and Drug Administration." Samsca (Tolvaptan): Drug Safety Communication. N.p., 30 Apr. 2013. Web. 1 June 2014. <http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm350185.htm>
- Gheorghiade M, Niazi I, Ouyang J, et al. (2003). "Vasopressin V2-receptor blockade with tolvaptan in patients with chronic heart failure: results from a double-blind, randomized trial". Circulation. 107 (21): 2690–6. doi:10.1161/01.CIR.0000070422.41439.04. PMID 12742979.