Diprotic acid
In chemistry, diprotic acid is a class of Arrhenius acids which are capable of donating two protons or hydrogen cations per molecule when dissociating in aqueous solutions.[1][2] The most important chemical feature for a diprotic acid molecule is its ability to deprotonate two protons in two sequential steps during dissociation. Most diprotic acids are common acids which are used everyday in many different areas. Also, they exist everywhere in nature, such as in human bodies.
General Structure
There are both organic (which are called dicarboxylic acids) and inorganic diprotic acids. Chromic acid (H2CrO4) and sulfuric acid (H2SO4) are two of the common and widely used inorganic acids. They have similar structures within each molecular has two -OH groups linked to its center atom.[5] Some other inorganic diprotic acids such as hydrosulfuric acid (H2S) usually have two hydrogen atoms linked to an electronegative center in each molecular.[6] Dicarboxylic acids have a general molecular formula HOOC-R-COOH.[7]
Dissociation and acid-dissociation equilibrium constants
A diprotic acid molecular dissociates in water and other aqueous solutions to be deprotonated. It forms two equilibrium step-by-step with two different equilibrium constants, which are also called acid-dissociation equilibrium constants. Usually,the constant for the first equilibrium is far larger than the second constant.
Dissociation in water
The dissociation equations of a diprotic acid in water can be written as:
-
H2A + H2O ⇌ H3O+ + HA− [8]
(1)
-
HA− + H2O ⇌ H3O+ + A2− [8]
(2)
The dissociation processes of a diprotic acid does not happen all at once due to the two stages of dissociation having different Ka values. Hence, each kind of diprtotic acid has two different acid-dissociation equilibrium constants, Ka1 and Ka2. The corresponding conjugate base of each conjugate acid state also has a different base-dissociation equilibrium constant Kb1 and Kb2, respectively.[9]
The constants Ka1 and Ka2 are defined at 25 °C in water[10] and can be written as:
Ka values for common diprotic acids
For most diprotic acids, the value of Ka1 is at least one hundred times larger than the value of Ka2.[13] This is mainly because that more energy is required to remove a positively charged proton from HA− with a negative charge in the above equation (2) than from H2A which is electric neutral in the above equation (1). This phenomena can also be demonstrated by Pauling's first rule.[14]
| Acid | Ka1 | Ka2 |
|---|---|---|
| sulfuric acid (H2SO4) | 1.0 x 103 | 1.2 x 10−2 |
| chromic acid (H2CrO4) | 9.6 | 3.2 x 10−7 |
| oxalic acid (H2C2O4) | 5.4 x 10−2 | 5.4 x 10−5 |
| sulfurous acid (H2SO3) | 1.7 x 10−2 | 6.4 x 10−8 |
| glycine (C2H6NO2) | 4.5 x 10−3 | 2.5 x 10−10 |
| carbonic acid (H2CO3) | 4.5 x 10−7 | 4.7 x 10−11 |
| hydrogen sulfide (H2S) | 1.0 x 10−7 | 1.3 x 10−13 |
| malonic acid (H2C3H2O4) | 1.5 x 10−3 | 2.0 x 10−6 |
pH calculation
The pH value for every solution is defined as the same, where pH=-log[H3O+].[17] However, the determination of the concentration of H+ in a diprotic acid solution is more complex than that of a monoprotic acid due to the two steps of dissociation of a diprotic acid which have correlations.
Solution of H2A
For an aqueous solution prepared by adding dipotic acid with initial concentration [H2A]=F,the pH value at equilibrium can be calculated by simply treating it as a monoprotic acid if Ka1 is greater than Ka2 by a factor of 103 or larger.[18] Then we can apply the equation (1):
-
(1)
For [H+]=[HA−] at equilibrium, we can assume that [H+]=[HA−]=x and then the equation (1) can be written as:
Note if the diprotic acid is a weak acid which has a Ka1 value ≤10−3, the equation above can be further simplified as:
By solving for x, we obtain the final concentration of the hydrogen ion of the solution. Finally, we can calculate the pH value by substituting x=[H+] into pH definition:[19]
Diprotic Buffers
A buffer made by a diprotic acid and its conjugate base can be treated in the same way as a buffer made by a monoprotic acid.[20] We can write two Henderson–Hasselbalch equations:
Depending on the quantity we know, we can either substituting [HA−] and [H2A] or [HA−] and [A−] to calculate the pH value of the buffer.[22]
Titration
To determine the concentration of a diprotic acid in an aqueous solution, an acid-base titration is commonly performed. A strong base solution with a known concentration, usually NaOH or KOH, is added to neutralize the acid solution.[23] According to the color change of the indicator with the amount of base added,
Titration curves
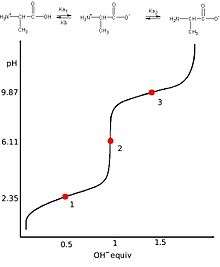
A titration curve of a diprotic acid titrated by a base has two axis, with the base volume on the x-axis and the solution's pH value on the y-axis.The pH of the solution always goes up as the base is added to the solution. For each diprotic acid titration curve, from left to right, there are two midpoints, two equivalence points, and two buffer regions.[24]
Equivalence points
Due to the successive dissociation processes, there are two equivalence points in the titration curve of a diprotic acid.[25] The first equivalence point occurs when all first hydrogen ions from the first ionization are titrated.[26] In other words, the amount of OH− added equals the original amount of H2A at the first equivalence point. The second equivalence point occurs when all hydrogen ions are titrated. Therefore, the amount of OH− added equals the twice the amount of H2A at this time. For a weak diprotic acid titrated by a strong base, the second equivalence point must occur at pH>7 due to the hydrolysis of the resulted salts in the solution.[27] At either equivalence point,adding a drop of base will cause the steepest rise of the pH value in the system.
Buffer regions and mid points
A titration curve for a diprotic acid contains two midpoints where pH=pKa. Since there are two different Ka values, the first midpoint occurs at pH=pKa1 and the second one occurs at pH=pKa2.[28] Each segment of the curve which contains a midpoint at its center is called the buffer region. Because the buffer regions consist of the acid and its conjugate base,it can resist pH changes when base is added until the next equivalent points.[29]
Diprotic acids in daily life
Diprotic acids account for a great part of acids and they exist universally in our life. There are both numerous kinds of natural dirpotic acid compounds with biological functions and massive synthesized diprotic acids which are used in many ways.
In human bodies
Our bodies contain a variety of organic and inorganic compounds, among those dicarboxylic acids play an essential role in many biological behaviors. Many of those diprotic acids are amino acids which mainly serve as materials for synthesis of proteins.[30] Other weak diprotic acids serve as body buffer with their conjugate bases to keep human body's H+ concentration from great changes which will be harmful to cells.[31] The rest of the dicarboxylic acids also participate in synthesis of various biologically important compounds in human bodies.
In industry
Acids are fundamental reagents in treating almost all processes in today's industry. Sulfuric acid, a diprotic acid, is the most widely used acid in industry, which is also the most-produced industrial chemical in the world. It is mainly used in producing fertilizes, detergent, batteries and dyes, as well as used in processing many products such like removing impurities.[32] According to the statistics data in 2011, the annual production of sulfuric acid was around 200 million tonnes in the world.[33]
In food
Many diprotic acids can be found in various kinds of food which is considered to be slightly acidic (pH<7).Some of the acids naturally existed in food and others are artificial addictive. Carbonic acid is one of the most common diprotic acid addictive that is widely added in soft drinks, such as Coca-Cola. During the manufacturing process of soft drinks, CO2 is usually pressurized to dissolve in these drinks to generate carbonic acid. Carbonic acid is very unstable and tend to decompose into water and CO2 in normal temperature and pressure. Therefore, when we open the bottles or cans of these kinds of soft drinks, CO2 bubbles come out and thus we feel 'sparks'.[34]
See also
References
- ↑ "Diprotic Acid Definition". About.com Education. Retrieved 2016-01-22.
- ↑ "What is a Diprotic Acid?". wiseGEEK. Retrieved 2016-01-23.
- ↑ Choe, Yoong-Kee; Tsuchida, Eiji; Ikeshoji, Tamio (2007-04-21). "First-principles molecular dynamics study on aqueous sulfuric acid solutions". The Journal of Chemical Physics. 126 (15): 154510. doi:10.1063/1.2718526. ISSN 0021-9606.
- ↑ Atkins, Peter; Jones, Loretta (2007-08-01). Chemical Principles: The Quest for Insight. Macmillan. ISBN 9781429209656.
- ↑ Kuczkowski, Robert L.; Suenram, R. D.; Lovas, Frank J. "Microwave spectrum, structure, and dipole moment of sulfuric acid". Journal of the American Chemical Society. 103 (10): 2561–2566. doi:10.1021/ja00400a013.
- ↑ "CHEMINFO: Hydrogen sulfide". www.ccohs.ca. Retrieved 2016-01-23.
- ↑ L., Leiserowitz,; IUCr (1976-03-15). "Molecular packing modes. Carboxylic acids". scripts.iucr.org. doi:10.1107/S0567740876003968. Retrieved 2016-01-23.
- 1 2 "Chemistry Tutorial : Polyprotic Weak Acid Concepts". www.ausetute.com.au. Retrieved 2016-01-23.
- ↑ "Acids/Bases - MCAT Review". mcat-review.org. Retrieved 2016-01-23.
- ↑ hriver, D.F; Atkins, P.W. (1999). Inorganic Chemistry (3rd ed.). Oxford: Oxford University Press. ISBN 0-19-850331-8. Chapter 5: Acids and Bases.
- ↑ "pH of polyprotic acid/base solution". www.chembuddy.com. Retrieved 2016-01-23.
- ↑ "Polyprotic Acids & Bases - Chemwiki". chemwiki.ucdavis.edu. Retrieved 2016-01-23.
- ↑ Rhee, Jae Seong; Dasgupta, Purnendu K. (2002-05-01). "The second dissociation constant of sulfur dioxide.water". The Journal of Physical Chemistry. 89 (9): 1799–1804. doi:10.1021/j100255a052.
- ↑ Greenwood, N.N. (1997). Chemistry of the Elements. Oxford: Butterworth-Heinemann. p. 50. ISBN 0-7506-3365-4.
- ↑ "Polyprotic Acids And Bases - Chemwiki". chemwiki.ucdavis.edu. Retrieved 2016-01-23.
- ↑ "Diprotic and Triprotic Acids and Bases". chemed.chem.purdue.edu. Retrieved 2016-01-23.
- ↑ "Determining and Calculating pH - Chemwiki". chemwiki.ucdavis.edu. Retrieved 2016-02-06.
- ↑ King, D. Whitney; Kester, Dana R. "A general approach for calculating polyprotic acid speciation and buffer capacity". Journal of Chemical Education. 67 (11). doi:10.1021/ed067p932.
- ↑ Covington, A. K.; Bates; Durst (1985). Definitions of pH scales, standard reference values, measurement of pH, and related terminology (PDF). 57. http://www.iupac.org/publications/pac/1985/pdf/5703x0531.pdf. pp. 531–542. doi:10.1351/pac198557030531.
- ↑ Harris, Daniel C. (2010-04-30). Quantitative Chemical Analysis. Macmillan. p. 193. ISBN 9781429218153.
- ↑ Jameson, Reginald F. "Assignment of the proton-association constants for 3-(3,4-dihydroxyphenyl)alanine (L-dopa)". pubs.rsc.org. doi:10.1039/DT9780000043. Retrieved 2016-01-29.
- ↑ Harris, Daniel C. (2003-01-01). Quantitative Chemical Analysis, Sixth Edition. Macmillan. p. 212. ISBN 9780716744641.
- ↑ Robert De, Levie (1999). Aqueous Acid-Base Equilibria and Titrations. New York: Oxford University Press.
- ↑ Helfferich, Friedrich G. (1962-01-01). Ion Exchange. Courier Corporation. ISBN 9780486687841.
- ↑ "Titration of Diprotic Acid". dwb.unl.edu. Retrieved 2016-01-24.
- ↑ Kotz, John C.; Treichel, Paul M.; Townsend, John; Treichel, David (2014-01-24). Chemistry & Chemical Reactivity. Cengage Learning. ISBN 9781305176461.
- ↑ Kotz, John; Treichel, Paul; Townsend, John (2009-02-09). Chemistry and Chemical Reactivity, Enhanced Edition. Cengage Learning. ISBN 0495390291.
- ↑ Lehninger, Albert L.; Nelson, David L.; Cox, Michael M. (2005-01-01). Lehninger Principles of Biochemistry. Macmillan. ISBN 9780716743392.
- ↑ Ebbing, Darrell; Gammon, Steven D. (2016-01-01). General Chemistry. Cengage Learning. ISBN 9781305887299.
- ↑ "8 - Biological roles of amino acids and peptides - University Publishing Online". ebooks.cambridge.org. Retrieved 2016-02-06.
- ↑ Graham, Timur (2006). "Acid Buffering". Acid Base Online Tutorial. University of Connecticut. Retrieved 2016-02-06.
- ↑ "The Top 10 Industrial Chemicals - For Dummies". www.dummies.com. Retrieved 2016-02-05.
- ↑ "Sulfuric acid". www.essentialchemicalindustry.org. Retrieved 2016-02-06.
- ↑ McMillin, John R.; Tracy, Gene A.; Harvill, William A.; Jr, William S. Credle (Dec 8, 1981), Method of and apparatus for making and dispensing a carbonated beverage utilizing propellant carbon dioxide gas for carbonating, retrieved 2016-02-06
External links
•Bodner Research Web:Diprotic and Triprotic Acids and Bases