Enantiopure drug
An enantiopure drug is a pharmaceutical that is available in one specific enantiomeric form. Most biological molecules (proteins, sugars, etc.) are present in only one of many chiral forms, so different enantiomers of a chiral drug molecule bind differently (or not at all) to target receptors. One enantiomer of a drug may have a desired beneficial effect while the other may cause serious and undesired side effects, or sometimes even beneficial but entirely different effects.[1] Advances in industrial chemical processes have made it economical for pharmaceutical manufacturers to take drugs that were originally marketed as a racemic mixture and market the individual enantiomers, either by specifically manufacturing the desired enantiomer or by resolving a racemic mixture. On a case-by-case basis, the U.S. Food and Drug Administration (FDA) has allowed single enantiomers of certain drugs to be marketed under a different name than the racemic mixture.[2] Also case-by-case, the United States Patent Office has granted patents for single enantiomers of certain drugs. The regulatory review for marketing approval (safety and efficacy) and for patenting (proprietary rights) is independent, and differs country by country.
Importance
Selectivity is a very important part of organic synthesis. In scientific papers regarding synthesis, selectivity is often listed in data tables alongside percent yield and other reaction conditions. While selectivity is deemed important in scientific literature, it has been challenging to effectively implement selectivity in drug development and production. A major issue with selectivity in pharmaceuticals is that a large percentage of drug syntheses by nature are not selective reactions, racemic mixtures are formed as the products. Separating racemic mixtures into their respective enantiomers takes extra time, money, and energy. One way to separate enantiomers is to chemically convert them into species that can be separated: diastereomers. Diastereomers, unlike enantiomers, have entirely different physical properties—boiling points, melting points, NMR shifts, solubilities—and they can be separated by conventional means such as chromatography or recrystallization. This is a whole extra step in the synthesis process and not desirable from a manufacturing standpoint. [3] As a result, a number of pharmaceuticals are synthesized and marketed as a racemic mixture of enantiomers. Being a racemic mixture of enantiomers does not put the consumer is any sort of danger, however; it is believed that by isolating a single enantiomer the effectiveness of a certain drug could be magnified. By finding the enantiomer which effectively binds to its respective binding site in the body, less of the drug would be needed to achieve the desired effect.[4]
Criteria
According to the FDA, the stereoisomeric composition of a chiral drug should be known, and its effects should be well-characterized from pharmacologic, toxicologic, and clinical standpoints. In order to profile the different stereoisomers of enantiopure drugs, manufacturers are urged to develop quantitative assays for individual enantiomers in in vivo samples early in the development stage.
Ideally, the main pharmacologic activities of the isomers should be compared in in vitro systems in animals. During instances when toxic findings are present beyond the natural extensions o the pharmacologic effects of the drug, toxicologic evaluation of the individual isomers in question must be completed.[5]
Patenting
When drugs are covered under patent protection, only the pharmaceutical company that holds the patent is allowed to manufacture, market, and eventually make profit from them. The lifetime of the patent varies between countries and also between drugs; in the United States, most drug patents last about twenty years.[6] Once the patent has expired, the drug can be manufactured and sold by other companies - at which point, it is referred to as a generic drug. Its availability on the market as a generic drug removes the monopoly of the patent holder, thereby encouraging competition and causing a significant drop in drug costs, which ensures that life-saving and important drugs reach the general population at fair prices. However, the company holding the initial patent may renew the patent by forming a new version of the drug that is significantly changed compared to the original compound.[7] Patentability of different isomers has been controversial over the past ten years and there have been a number of related legal issues. In making their determinations, courts have looked at factors including: (i) whether the racemate was known in the prior art; (ii) the difficulty in resolving the enantiomer; (iii) the stereoselectivity of the relevant receptor; and (iv) other secondary considerations of non-obviousness such as commercial success, unexpected results, and satisfaction of long-felt needs in the art. The decisions made regarding these issues have varied and there is no clear answer to the legality of patenting stereoisomers. These issues have been resolved on a case by case basis.[8] With the number of current pharmaceuticals currently being marketed as racemic mixtures, it is likely that patentability will continue to be debated in the near future.
Examples
The following table lists pharmaceuticals that have been available in both racemic and single-enantiomer form.
| Racemic mixture | Single-enantiomer |
|---|---|
| Amlodipine (Norvasc) | Levamlodipine (EsCordi Cor) |
| Amphetamine (Benzedrine) | Dextroamphetamine (Dexedrine) |
| Bupivacaine (Marcain) | Levobupivacaine (Chirocaine) |
| Cetirizine (Zyrtec / Reactine) | Levocetirizine (Xyzal) |
| Chlorphenamine (INN) Chlorpheniramine (USAN) (Chlor-Trimeton) |
Dexchlorpheniramine (Polaramine) |
| Citalopram (Celexa / Cipramil) | Escitalopram (Lexapro / Cipralex) |
| Fenfluramine (Pondimin) | Dexfenfluramine (Redux) |
| Formoterol (Foradil) | Arformoterol (Brovana) |
| Ibuprofen (Advil / Motrin) | Dexibuprofen (Seractil) |
| Ketamine (Ketalar) | Esketamine (Ketanest S) |
| Ketoprofen (Actron) | Dexketoprofen (Keral) |
| Methylphenidate (Ritalin) | Dexmethylphenidate (Focalin) |
| Milnacipran (Ixel / Savella) | Levomilnacipran (Fetzima) |
| Modafinil (Provigil) | Armodafinil (Nuvigil) |
| Ofloxacin (Floxin) | Levofloxacin (Levaquin) |
| Omeprazole (Prilosec) | Esomeprazole (Nexium) |
| Salbutamol (Ventolin) | Levalbuterol (Xopenex) |
| Zopiclone (Imovane / Zimovane) | Eszopiclone (Lunesta) |
The following are cases where the individual enantiomers have markedly different effects:

- Thalidomide: Thalidomide is racemic. One enantiomer is effective against morning sickness, whereas the other is teratogenic. However, the enantiomers are converted into each other in vivo.[9] As a result, dosing with a single-enantiomer form of the drug will still lead to both the enantiomers eventually being present in the patient's serum and thus would not prevent adverse effects—at best, it might reduce them if the rate of in vivo conversion can be slowed.[10]
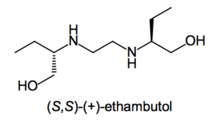
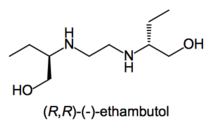
- Ethambutol: Whereas the (S,S)-(+)-enantiomer is used to treat tuberculosis, the (R,R)-(–)-ethambutol causes blindness.[11]
- Naproxen: (S)-(+)-naproxen is used to treat arthritis pain, but (R)-(–)-naproxen causes liver poisoning with no analgesic effect.
- Steroid receptor sites also show stereoisomer specificity.
- Penicillin's activity is stereodependent. The antibiotic must mimic the D-alanine chains that occur in the cell walls of bacteria in order to react with and subsequently inhibit bacterial transpeptidase enzyme.
- Propranolol: L-propranolol is a powerful adrenoceptor antagonist, whereas D-propranolol is not. However, both have local anesthetic effect.
- Methorphan: The L-isomer of methorphan, levomethorphan, is a potent opioid analgesic, while the D-isomer, dextromethorphan, is a dissociative cough suppressant.
- Carvedilol: (S)-(–)-isomer interacts with adrenoceptors with 100 times greater potency as β adrenoreceptor blocker than (R)-(+)-isomer. However, both the isomers are approximately equipotent as α adrenoreceptor blockers.
- Amphetamine and methamphetamine: The D-isomers of these drugs are strong central nervous system (CNS) stimulants, while the L-isomers lack appreciable CNS stimulant effects, but instead stimulate the peripheral nervous system. For this reason, the L-isomer of methamphetamine is available as an over-the-counter nasal inhaler in some countries, while the D-isomer is banned from medical use in all but a few countries in the world, and highly regulated in those countries which do allow it to be used medically.
-ketamine-from-xtal-3D-balls.png)

- Ketamine: This drug is available as a mixture of both (S)-(–)-ketamine, also known as esketamine, and (R)-(–)-ketamine, also known as arketamine. Pure esketamine is also available. The two have different dissociative and hallucinogenic properties, with esketamine being more potent in isolation as a dissociative.[12] The two enantiomers have inverse effects on the rate of glucose metabolism in the frontal cortex.[13]
See also
References
- ↑ E. J. Ariëns: Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology European Journal of Clinical Pharmacology 26 (1984) 663-668. doi:10.1007/BF00541922 Preview:
- ↑ US FDA's policy statement on the development of new stereoisomeric drugs
- ↑ Separation of Enantiomers http://www.chemhelper.com/enantiomersep.html. Retrieved 2016-04-13.
- ↑ Owens, Michael. "Stereochemistry in Drug Action". US National Library of Medicine National Institutes of Health. pp. 70–73. Retrieved 16 April 2016.
- ↑ Research, Center for Drug Evaluation and. "Guidances (Drugs) - Development of New Stereoisomeric Drugs". www.fda.gov. Retrieved 2016-03-15.
- ↑ Research, Center for Drug Evaluation and. "Development & Approval Process (Drugs) - Frequently Asked Questions on Patents and Exclusivity". www.fda.gov. Retrieved 2016-03-16.
- ↑ "Drug Patents and Generic Pharmaceutical Drugs". News-Medical.net. Retrieved 2016-03-16.
- ↑ Sodikoff, Brian. "Enantiomer Patents: Innovative or Obvious?" (PDF). Kattenlaw. Retrieved 16 April 2016.
- ↑ Teo SK, Colburn WA, Tracewell WG, Kook KA, Stirling DI, Jaworsky MS, Scheffler MA, Thomas SD, Laskin OL (2004). "Clinical pharmacokinetics of thalidomide". Clin Pharmacokinet. 43 (5): 311–327. doi:10.2165/00003088-200443050-00004. PMID 15080764.
- ↑ Smith, Silas. "Chiral Toxicology: It's the Same Thing... Only Different". Toxicology Sciences. pp. 4–30. doi:10.1093/toxsci/kfp097. Retrieved 16 April 2016.
- ↑ Padmanabhan, Deepak. "A review of drug isomerism and its significance". US National Library of Medicine National Institutes of Health. pp. 16–18. doi:10.4103/2229-516X.112233. Retrieved 16 April 2016.
- ↑ Trevor, AJ. "Comparative Pharmacology of the ketamine isomers. Studies in volunteers.". US National Library of Medicine National Institutes of Health. pp. 197–203. Retrieved 16 April 2016.
- ↑ Vollenweider, F. X.; Leenders, K. L.; Oye, I.; Hell, D.; Angst, J. (1997). "Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET)". European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 7 (1): 25–38. doi:10.1016/S0924-977X(96)00042-9. PMID 9088882.