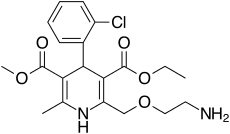
Amlodipine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Norvasc, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692044 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth (tablets) |
| ATC code | C08CA01 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 64 to 90% |
| Metabolism | Liver |
| Biological half-life | 30 h to 50 h |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number |
88150-42-9 |
| PubChem (CID) | 2162 |
| IUPHAR/BPS | 6981 |
| DrugBank |
DB00381 |
| ChemSpider |
2077 |
| UNII |
1J444QC288 |
| KEGG |
D07450 |
| ChEBI |
CHEBI:2668 |
| ChEMBL |
CHEMBL1491 |
| PDB ligand ID | 6UB (PDBe, RCSB PDB) |
| Chemical and physical data | |
| Formula | C20H25ClN2O5 |
| Molar mass | 408.879 g/mol |
| 3D model (Jmol) | Interactive image |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Amlodipine, sold under the brand name Norvasc among others, is a medication used to treat high blood pressure and coronary artery disease.[1] While calcium channel blockers are not typically recommended in heart failure, amlodipine may be used if other medications are not sufficient for high blood pressure or heart related chest pain.[2] Amlodipine is taken by mouth and has an effect for at least a day.[1]
Common side effects include: swelling, feeling tired, abdominal pain, and nausea. Serious side effects may include low blood pressure or a heart attack. It is unclear if use is safe during pregnancy or breastfeeding. Doses should be decreased in people with liver problems and those who are old. Amlodipine is a long acting calcium channel blocker of the dihydropyridine type. It works partly by increasing the size of arteries.[1]
Amlodipine was first patented in 1986 with commercial sale beginning in 1990.[3] It is on the WHO Model List of Essential Medicines, the most important medications needed in a basic health system.[4] It is available as a generic medication.[1] Wholesale cost in the developing world is 0.003 to 0.040 USD per day for a typical dose as of 2014.[5] In the United States a months supply costs less than 25 USD.[6]
Medical uses
Amlodipine is used in the management of hypertension[7] and coronary artery disease (chronic stable angina, vasospastic angina, and angiographically documented CAD without heart failure or ejection fraction < 40%.)[8] It can be used as either monotherapy or combination therapy for the management of hypertension or coronary artery disease.[8] Amlodipine can be administered to adults and children 6–17 years of age.[8]
Contraindications
Absolute
Relative
- Cardiogenic shock[9]
- Unstable angina[9]
- Systolic and diastolic blood pressure below 90/60 mmHg[9]
- Aortic stenosis[9]
- Breastfeeding[9]
- Pregnancy[9]
Adverse effects
Adverse side effects of the use of amlodipine may include:[9][10]
- Common and dose-related: peripheral edema (5.1%), dizziness (2.6%), palpitations (2.1%), flushing (1.5%)
- Common, not dose-related: fatigue (4.5%), nausea (2.9%), abdominal pain (1.6%), somnolence (1.4%)
- Rare (less than 1% incidence): blood disorders, impotence, depression, insomnia, tachycardia, or gingival enlargement, hepatitis, jaundice
Overdosage
Although rare,[11] amlodipine overdose toxicity can result in widening of blood vessels, severe low blood pressure, and fast heart rate.[12][13]
Amlodipine toxicity is generally managed with the following measures:
- Fluid replacement[12][13][14]
- Monitoring of ECG, vitals, respiratory system, glucose, kidney function, electrolytes, and urine output[12][13]
- Vasopressor administration (for low blood pressure unresponsive to fluid resuscitation)[12]
Mechanism of action
Amlodipine is an angioselective calcium channel blocker. Calcium stimulates the actions of cells. Calcium channels are found on heart muscles, blood vessels, and other cells. When the channels open, calcium flows into the cell, causing them to contract. Calcium channel blockers interfere with this stimulation.[1] This relaxes blood vessels widening them.[1] This lowers blood pressure and reduces the stress on the heart.[1] In those with heart related chest pain, amlodipine may increase blood flow to the heart muscle.[1]
Amlodipine inhibits the movement of calcium ions into vascular smooth muscle cells and cardiac muscle cells. The contractile processes of cardiac muscle and vascular smooth muscle are dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. Amlodipine inhibits calcium ion influx across cell membranes selectively, with a greater effect on vascular smooth muscle cells. Negative inotropic effects can be detected in vitro, but such effects have not been seen in intact animals at therapeutic doses. Among two sterioisomers [R(+), S(-)], the (-) isomer has been reported to be more active than the (+) isomer.[15]Serum calcium concentration is not affected by amlodipine. Amlodipine is a peripheral arterial vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and reduction in blood pressure. As a calcium channel blocker, amlodipine is expected to inhibit the currents of L-type Cav1.3 channels in the zona glomerulosa.[16][17]
The mechanisms by which amlodipine relieves angina include:
- Stable angina: amlodipine reduces the total peripheral resistance (afterload) against which the heart works and reduces the rate pressure product, thereby lowering myocardial oxygen demand, at any given level of exercise.[18]
- Prinzmetal's angina: amlodipine blocks spasm of the coronary arteries and restores blood flow in coronary arteries and arterioles in response to calcium, potassium, epinephrine, serotonin, and thromboxane A2 analog in experimental animal models and in human coronary vessels in vitro.[19]
Amlodipine has additionally been found to act as an antagonist of the mineralocorticoid receptor, or as an antimineralocorticoid.[20]
Metabolism
Amlodipine has been studied in healthy volunteers following oral administration of 14C-labelled drug.[21] amlodipine is well absorbed by the oral route with a mean oral bioavailability around 60%. It is metabolized in the liver to inactive metabolites via CYP3A4. The half-life of amlodipine is about 30 h to 50 h, and steady-state plasma concentrations are achieved after 7 to 8 days of daily dosing. Renal elimination is the major route of excretion with about 60% of an administered dose recovered in urine, largely as inactive pyridine metabolites. However, renal impairment does not significantly influence amlodipine elimination.[22]
Interactions
- CYP3A inhibitors: giving with moderate to strong inhibitors of the cytochrome p450 enzyme CYP3A4 (such as itraconazole, clarithromycin) that metabolizes amlodipine in the liver may increase the plasma concentrations of amlodipine.[23]
- CYP3A inducers: No data is currently available for co-administration of CYP3A4 inducers with amlodipine.[23]
- Simvastatin: giving with simvastatin at doses greater than 20 mg can lead to an increase in simvastatin plasma concentrations and increase risk of myopathy.[24]
- Sildenafil: giving with sildenafil can lead to an increased risk of hypotension.[23]
- Tacrolimus: giving with tacrolimus in patients with the CYP3A5*3 genetic polymorphism may increase tacrolimus plasma concentrations.[25]
Combination therapy
If monotherapy with amlodipine or candesartan is not sufficient to reach the reducing blood pressure target, a combination of amlodipine and candesartan can be effective, lowering blood pressure after 12 weeks in people not adequately controlled by monotherapy. People suffering from high blood pressure and high cholesterol can also benefit from using a combination of amlodipine with atorvastatin.[26]
History
Pfizer's patent protection on Norvasc lasted until 2007; total patent expiration occurred later in 2007.[27] A number of generic versions are available. In the United Kingdom, tablets of amlodipine from different suppliers may contain different salts. The strength of the tablets is expressed in terms of amlodipine base, i.e., without the salts. Tablets containing different salts are therefore considered interchangeable. The efficacy and tolerability of a fixed-dose combination of amlodipine and perindopril, an angiotensin converting enzyme inhibitor, have recently been confirmed in a prospective, observational, multicentre trial of 1250 hypertensive patients.[28]
The medical form comes as besylate, mesylate or maleate.[29]
References
- 1 2 3 4 5 6 7 8 "Amlodipine Besylate". Drugs.com. American Society of Hospital Pharmacists. Retrieved 22 July 2016.
- ↑ The ESC Textbook of Preventive Cardiology: Clinical Practice. Oxford University Press. 2015. p. 261. ISBN 9780199656653.
- ↑ Chorghade, Mukund S. (2006). Drug Discovery and Development Volume 1. Hoboken: John Wiley & Sons. p. 207. ISBN 9780471780090. Retrieved 23 July 2016.
- ↑ "19th WHO Model List of Essential Medicines (April 2015)" (PDF). WHO. April 2015. Retrieved May 10, 2015.
- ↑ "Amlodipine". International Drug Price Indicator Guide. Retrieved 23 July 2016.
- ↑ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 154. ISBN 9781284057560.
- ↑ Wang, JG (2009). "A combined role of calcium channel blockers and angiotensin receptor blockers in stroke prevention". Vascular health and risk management. 5: 593–605. doi:10.2147/vhrm.s6203. PMID 19688100.
- 1 2 3 "Norvasc Prescribing Information" (PDF).
- 1 2 3 4 5 6 7 8 "DailyMed - AMLODIPINE - amlodipine besylate tablet". dailymed.nlm.nih.gov. Retrieved 2015-11-05.
- ↑ Munoz, Ricardo; Vetterly, Carol G.; Roth, Stephen J.; Cruz, Eduardo da (2007-10-18). Handbook of Pediatric Cardiovascular Drugs. Springer Science & Business Media. ISBN 9781846289538.
- ↑ Aronson, J (2014). Side Effects of Drugs Annual 35. Elsevier. ISBN 978-0-444-62635-6.
- 1 2 3 4 "Norvasc tablets label-FDA" (PDF). Retrieved November 2, 2015.
- 1 2 3 Pillay, V (2013). Modern Medical Toxicology 4th Ed. Jaypee. ISBN 978-93-5025-965-8.
- ↑ Hui, David (2015). Approach to Internal Medicine: A Resource Book for Clinical Practice Fourth Edition. Springer. ISBN 978-3-319-11820-8.
- ↑ Karmoker, J.R.; Joydhar, P.; Sarkar, S.; Rahman, M. (2016). "Comparative in vitro evaluation of various commercial brands of amlodipine besylate tablets marketed in Bangladesh" (PDF). Asian Journal of Pharmaceutical and Health Sciences. 6: 1384–1389. Retrieved 2016-05-29.
- ↑ Arcangelo, Virginia Poole; Peterson, Andrew M. (2006). Pharmacotherapeutics for Advanced Practice: A Practical Approach. Lippincott Williams & Wilkins. ISBN 9780781757843.
- ↑ Ritter, James; Lewis, Lionel; Mant, Timothy; Ferro, Albert (2012-12-11). A Textbook of Clinical Pharmacology and Therapeutics, 5Ed. CRC Press. ISBN 9781444113006.
- ↑ Li, Y. Robert (2015-04-06). Cardiovascular Diseases: From Molecular Pharmacology to Evidence-Based Therapeutics. John Wiley & Sons. ISBN 9780470915370.
- ↑ LEARNING, JONES & BARTLETT; Bartlett, Jones and (2012-07-15). 2013 Nurse's Drug Handbook. Jones & Bartlett Publishers. ISBN 9781449642846.
- ↑ Luther, James M. (2014). "Is there a new dawn for selective mineralocorticoid receptor antagonism?". Current Opinion in Nephrology and Hypertension. 23 (5): 456–461. doi:10.1097/MNH.0000000000000051. ISSN 1062-4821.
- ↑ Beresford AP, McGibney D, Humphrey MJ, Macrae PV, Stopher DA (February 1988). "Metabolism and kinetics of amlodipine in man". Xenobiotica. 18 (2): 245–54. doi:10.3109/00498258809041660. PMID 2967593.
- ↑ Brittain, Harry G. (2012-05-09). Profiles of Drug Substances, Excipients and Related Methodology. Academic Press. ISBN 9780123977564.
- 1 2 3 "Pfizer Laboratories Div Pfizer Inc".
- ↑ Research, Center for Drug Evaluation and. "Drug Safety and Availability - FDA Drug Safety Communication: New restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury". www.fda.gov. Retrieved 2015-11-05.
- ↑ Zuo, Xiao-cong; Zhou, Ya-nan; Zhang, Bi-kui; Yang, Guo-ping; Cheng, Ze-neng; Yuan, Hong; Ouyang, Dong-sheng; Liu, Shi-kun; Barrett, Jeffrey S. (2013-01-01). "Effect of CYP3A5*3 Polymorphism on Pharmacokinetic Drug Interaction between Tacrolimus and Amlodipine". Drug Metabolism and Pharmacokinetics. 28 (5): 398–405. doi:10.2133/dmpk.DMPK-12-RG-148.
- ↑ Delgado-Montero, Antonia; Zamorano, Jose L. (2012-12-01). "Atorvastatin calcium plus amlodipine for the treatment of hypertension". Expert Opinion on Pharmacotherapy. 13 (18): 2673–2685. doi:10.1517/14656566.2012.742064. ISSN 1465-6566.
- ↑ Kennedy VB (22 March 2007). "Pfizer loses court ruling on Norvasc patent". MarketWatch.
- ↑ Bahl VK, Jadhav UM, Thacker HP (2009). "Management of hypertension with the fixed combination of perindopril and amlodipine in daily clinical practice: results from the STRONG prospective, observational, multicenter study". Am J Cardiovasc Drugs. 9 (3): 135–42. doi:10.2165/00129784-200909030-00001. PMID 19463019.
- ↑ Campbell, Anthony K. Intracellular Calcium. John Wiley & Sons. p. 68. ISBN 9781118675526. Retrieved 10 August 2016.