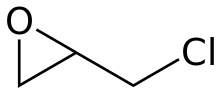
Epichlorohydrin
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-(Chloromethyl)oxirane | |
| Other names
(Chloromethyl)oxirane Epichlorohydrin 1-Chloro-2,3-epoxypropane γ-Chloropropylene oxide Glycidyl chloride | |
| Identifiers | |
| 106-89-8 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:37144 |
| ChemSpider | 13837112 |
| ECHA InfoCard | 100.003.128 |
| KEGG | C14449 |
| PubChem | 7835 |
| UNII | 08OOR508C0 |
| |
| |
| Properties | |
| C3H5ClO | |
| Molar mass | 92.52 g/mol |
| Appearance | colorless liquid |
| Odor | garlic or chloroform-like |
| Density | 1.1812 g/cm3 |
| Melting point | −25.6 °C (−14.1 °F; 247.6 K) |
| Boiling point | 117.9 °C (244.2 °F; 391.0 K) |
| 7% (20°C)[2] | |
| Vapor pressure | 13 mmHg (20°C)[2] |
| Hazards | |
| Safety data sheet | External MSDS |
| EU classification (DSD) |
|
| NFPA 704 | |
| Flash point | 32 °C (90 °F; 305 K) |
| Explosive limits | 3.8%-21%[2] |
| Lethal dose or concentration (LD, LC): | |
| LC50 (median concentration) |
3617 ppm (rat, 1 hr) 2165 ppm (rat, 1 hr) 250 ppm (rat, 8 hr) 244 ppm (rat, 8 hr) 360 ppm (rat, 6 hr)[3] |
| LCLo (lowest published) |
250 ppm (rat, 4 hr)[3] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 5 ppm (19 mg/m3) [skin][2] |
| REL (Recommended) |
Carcinogen[2] |
| IDLH (Immediate danger) |
Ca [75 ppm][2] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Epichlorohydrin (abbreviated ECH) is an organochlorine compound and an epoxide. Despite its name, it is not a halohydrin. It is a colorless liquid with a pungent, garlic-like odor, moderately soluble in water, but miscible with most polar organic solvents.[4] It is a chiral molecule generally existing as a racemic mixture of right-handed and left-handed enantiomers. Epichlorohydrin is a highly reactive compound and is used in the production of glycerol, plastics, epoxy glues and resins, and elastomers. In contact with water, epichlorohydrin hydrolyzes to 3-MCPD, a carcinogen found in food.
Production
Epichlorohydrin was first described in 1848 by Marcellin Berthelot. The compound was isolated during studies on reactions between glycerol and gaseous hydrogen chloride.[5]
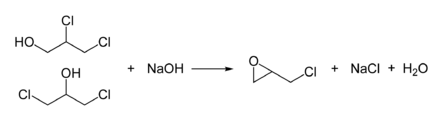
Epichlorohydrin is manufactured from allyl chloride in two steps, beginning with the addition of hypochlorous acid, which affords a mixture of two alcohols:[6][7]
- CH2=CHCH2Cl + HOCl → HOCH2CHClCH2Cl and, or ClCH2CH(OH)CH2Cl
In the second step, this mixture is treated with base to give the epoxide:
In this way, more than 800,000 tons (1997) of epichlorohydrin are produced annually.[8]
From glycerol
Glycerol is a co-product of biodiesel produced on a large scale. The conversion of glycerol into other building block chemicals is of interest because glycerol is otherwise hard to dispose of. Dow and Solvay are building glycerol-to-epichlorohydrin (GTE) plants in Shanghai and Thailand, respectively.[9] In Dow's process, glycerol is dichlorinated with hydrogen chloride with the help of a carboxylic acid catalyst. The mixture of dichlorohydroxypropanes is treated with base to form epichlorohydrin.[10]
Applications
Glycerol and epoxy resins synthesis
Epichlorohydrin is mainly converted to bisphenol A diglycidyl ether, a building block in the manufacture of epoxy resins. It is also a precursor to monomers for other resins and polymers. Another usage is the conversion to synthetic glycerol:
- CH2CHOCH2Cl + 2 H2O → HOCH2CH(OH)CH2(OH) + HCl
However, the rapid increase in biodiesel production, where glycerol is a waste product, has led to a glut of glycerol on the market, rendering this process uneconomic for the mass market. Synthetic glycerol is now used only in sensitive pharmaceutical, technical and personal care applications where quality standards are very high.[11]
Minor and niche applications
Epichlorohydrin is a versatile precursor in the synthesis of many organic compounds. For example, it is converted to glycidyl nitrate, an energetic binder used in explosive and propellant compositions.[12] The epichlorohydrin is reacted with an alkali nitrate, such as sodium nitrate, producing glycidyl nitrate and alkali chloride. It is used as a solvent for cellulose, resins, and paints, and it has found use as an insect fumigant.[13]
Polymers made from epichlorohydrin, e.g., polyamide-epichlorohydrin resins, are used in paper reinforcement and in the food industry to manufacture tea bags, coffee filters, and sausage/salami casings as well as with water purification.[14]
An important biochemical application of epichlorohydrin is its use as crosslinking agent for the production of Sephadex size-exclusion chromatographic resins from dextrans.[15]
Safety
Epichlorohydrin is classified by several international health research agencies and groups as a probable or likely human carcinogen in humans.[16][17][18] Prolonged oral consumption of high levels of epichlorohydrin could result in stomach problems and an increased risk of cancer.[19] Occupational exposure to epichlorohydrin via inhalation could result in lung irritation and an increased risk of lung cancer.[20]
See also
References
- ↑ Merck Index, 12th Edition, 3648.
- 1 2 3 4 5 6 "NIOSH Pocket Guide to Chemical Hazards #0254". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 "Epichlorohydrin". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ "EPA consumer factsheet". Epa.gov. Retrieved 2011-12-02.
- ↑ Berthelot, Marcellin (1854). "Sur les combinaisons de la glycérine avec les acides et sur la synthèse des principes immédiats des graisses animaux". Ann. Chim. Phys. SER3. 41: 216–319.
- ↑ Géza Braun "Epichlorohydrin and Epibromohydrin" Organic Syntheses,1936, Vol. 16, p.30. doi:10.15227/orgsyn.016.0030
- ↑ Guenter Sienel; Robert Rieth; Kenneth T. Rowbottom (2005), "Epoxides", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a09_531
- ↑ Ludger Krähling; Jürgen Krey; Gerald Jakobson; Johann Grolig; Leopold Miksche (2005), "Allyl Compounds", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a01_425
- ↑ Doris de Guzman (2011-01-20). "Growing glycerine-to-ECH plants". ICIS Green Chemicals.
- ↑ Bell, Bruce M.; Briggs, John R.; Campbell, Robert M.; Chambers, Susanne M.; Gaarenstroom, Phil D.; Hippler, Jeffrey G.; Hook, Bruce D.; Kearns, Kenneth; et al. (2008). "Glycerin as a Renewable Feedstock for Epichlorohydrin Production. The GTE Process" (full text reprint). CLEAN - Soil, Air, Water. 36 (8): 657. doi:10.1002/clen.200800067.
- ↑ "OPTIM Synthetic Glycerine". Retrieved 2012-03-05.
- ↑ Gould, R.F. Advanced Propellant Chemistry, ACS Chemistry Series 54, 1966
- ↑ "Suburban Water Testing Labs:Epichlorohydrin Fact Sheet". H2otest.com. Retrieved 2011-12-02.
- ↑ "Government of Canada Chemical Substances: Oxirane,(chloromethyl)-(Epichlorohydrin) CAS Registry Number 106-89-8". Retrieved 2013-05-07.
- ↑ "GE Healthcare Life Sciences - Instructions for Sephadex Media". .gelifesciences.com. Retrieved 2011-12-02.
- ↑ "EPA Integrated Risk Information System: Epichlorohydrin (CASRN 106-89-8)". Retrieved 2013-05-07.
- ↑ "Government of Canada: Screening Assessment for Epichlorohydrin". Retrieved 2013-05-07.
- ↑ "NIOSH Pocket Guide to Chemical Hazards - Epichlorohydrin". Retrieved 2013-09-20.
- ↑ "Basic Information about Epichlorohydrin in Drinking Water". Retrieved 2013-05-07.
- ↑ "Government of Canada: Screening Assessment for Epichlorohydrin". Retrieved 2013-05-07.