Formylation reaction

The formyl group
A formylation reaction in organic chemistry is the catch-all name for any organic reaction in which an organic compound is functionalized with a formyl group (-CH=O).
Aromatic formylation reactions via electrophilic aromatic substitution include:
- Dimethylformamide and phosphorus oxychloride in the Vilsmeier-Haack reaction
- Hexamine in the Duff reaction and the Sommelet reaction
- Carbon monoxide and hydrochloric acid in the Gattermann-Koch reaction
- Anionic cyanides in the Gattermann reaction. This method synthetizes aromatic aldehydes using hydrogen chloride and hydrogen cyanide (or another metallic cyanide as such zinc cyanide) in the presence of Lewis acid catalysts:
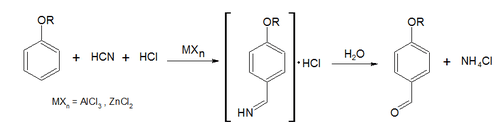
- Chloroform in the Reimer-Tiemann reaction
- dichloromethyl methyl ether in Rieche formylation
- Formylation of 3-methylamino-1-propanol with formamide (instead of ethyl chloroformate) as a protecting group in the preparation of Protriptyline. Formamide was also used (instead of formic acid) in the synthesis of primidone.
In biological systems, formylation is one of the processes of posttranslational modification in which a formyl group is added to the N-terminus of a protein.
See also
This article is issued from Wikipedia - version of the 7/9/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.