Fusaric acid
 | |
| Names | |
|---|---|
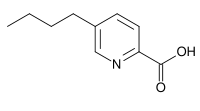
| Preferred IUPAC name
5-Butylpyridine-2-carboxylic acid | |
| Other names
5-Butylpicolinic acid Fusarinic acid | |
| Identifiers | |
| 536-69-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 3324 |
| ECHA InfoCard | 100.007.859 |
| EC Number | 208-643-0 |
| KEGG | C10146 |
| MeSH | D005669 |
| PubChem | 3442 |
| |
| |
| Properties | |
| C10H13NO2 | |
| Molar mass | 179.22 g·mol−1 |
| Melting point | 97 to 98 °C (207 to 208 °F; 370 to 371 K) |
| Related compounds | |
| Related compounds |
picolinic acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Fusaric acid is a picolinic acid derivative.
Antibiotic (wilting agent) first isolated from the fungus Fusarium heterosporium.[1]
It is typically isolated from various Fusarium species, and has been proposed for a various therapeutic applications. However, it is primarily used as a research tool.
Its mechanism of action is not well understood. It likely inhibits Dopamine beta-hydroxylase (the enzyme that converts dopamine to norepinephrine). It may also have other actions, such as the inhibition of cell proliferation and DNA synthesis. Fusaric acid and analogues also reported as quorum sensing inhibitors.[2]
It is used to make bupicomide.
References
This article is issued from Wikipedia - version of the 11/26/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.