Geminal
In chemistry, the term geminal refers to the relationship between two atoms or functional groups that are attached to the same atom. The word comes from Latin gemini meaning "twins".[1] A geminal diol, for example, is a diol (a molecule that has two alcohol functional groups) attached to the same carbon atom, as in methanediol.
The prefix gem may also be applied to a chemical name to denote this relationship, as in a gem-dibromide for "geminal dibromide".
The concept is important in many branches of chemistry, including synthesis and spectroscopy, because functional groups attached to the same atom often behave differently from when they are separated. Geminal diols, for example, are easily converted to ketones or aldehydes with loss of water.[2]
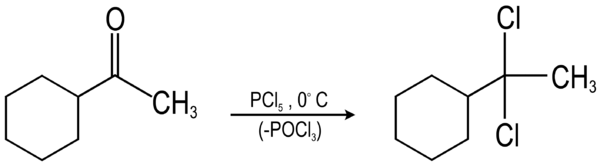
The following example shows the conversion of a cyclohexyl methyl ketone to a gem-dichloride through a reaction with phosphorus pentachloride. This gem-dichloride can then be used to synthesize an alkyne.
The related term vicinal refers to the relationship between two functional groups that are attached to adjacent atoms.
See also
References
- ↑ entry for "geminal" in the Oxford online dictionary of American English. Accessed on 2013-01-27.
- ↑ Peter Taylor (2002), Mechanism and synthesis, Book 10 of Molecular world. Open University, Royal Society of Chemistry; ISBN 0-85404-695-X. 368 pages.